On February 16, Iovance Biotherapeutics announced that the FDA had accelerated the approval of the company’s tumor-infiltrating lymphocyte (TIL) therapy Amtagvi (lifileucel) for the treatment of advanced melanoma. It is reported that lifileucel is the world's first TIL cell therapy approved for marketing.
 X
X-
This accelerated approval for marketing is mainly based on the results of the C-144-01 trial. C-144-01 is a global multi-center phase 2 study. The study is divided into 4 cohorts. See the figure below for details:
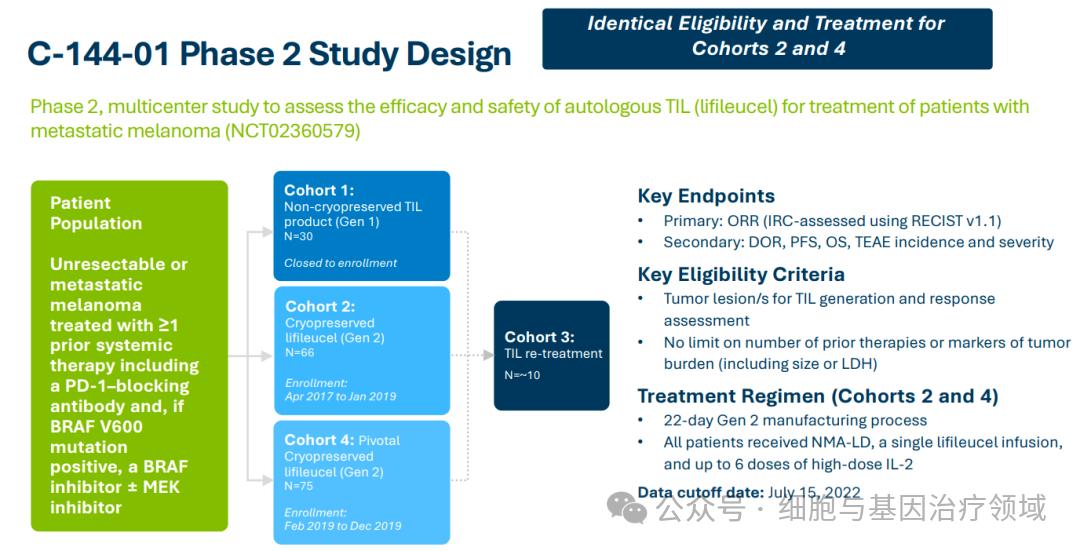 X
X- The results showed that the efficacy of lifileucel was relatively durable. At a median follow-up time of 27.6 months, in patients who had undergone extensive pretreatment (median number of lines of treatment: 3), the objective response rate assessed by the Independent Review Committee (IRC) was ( ORR) was 31.4%, including 8 patients who achieved complete response (CR) and 40 patients who achieved partial response (PR).

- In addition, clinical trial safety data are as follows:
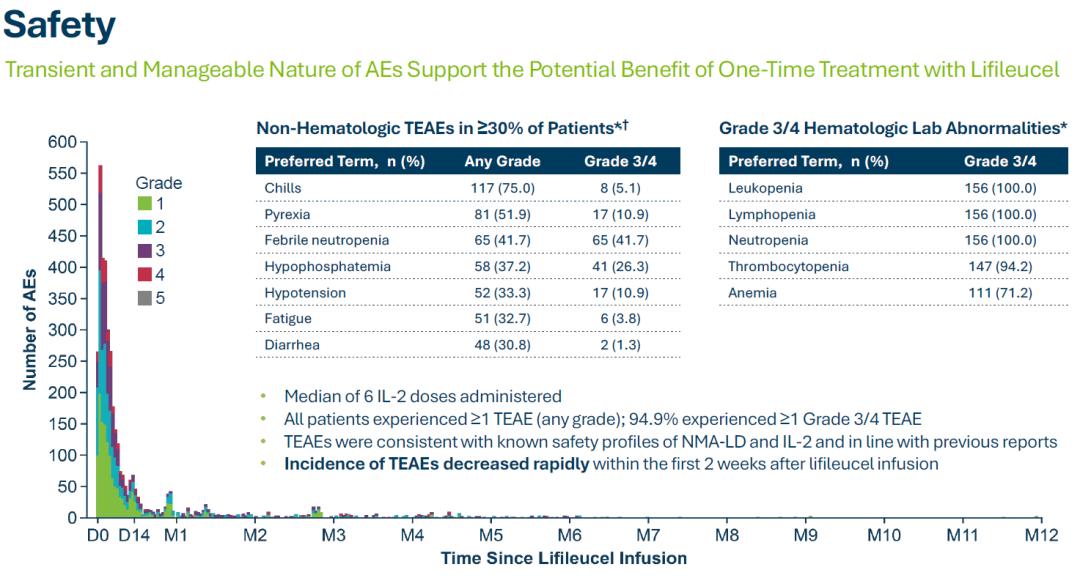
- TIL exists in the stroma of tumor tissue and is a heterogeneous cell population composed of CD8+T cells, CD4+T cells, B cells, NK cells and γδT cells. The direct killing effect on tumor cells is mainly through cytotoxicity. CD8+ T cells are achieved. However, due to the existence of an immunosuppressive microenvironment in tumor tissues, the expansion and anti-tumor activity of TILs in tumor tissues are often inhibited. TIL therapy is to isolate TIL from the patient's own tumor tissue, stimulate and amplify it in vitro, and then inject immune cells with anti-tumor effects back to the patient (see the figure below). Some data show that after in vitro stimulation, amplification and screening, the anti-tumor effect of TIL is more than 50 to 100 times higher than that of LAK cells (lymphokine-activated killer cells).
 X
X- TIL therapy process (image source: www.iovance.com)




