Medicinal excipients namely active drug is processed into a preparation, used in the prescription of excipients and additives, its inactive, is inert, but it is also a functional group of organic compounds, chemical reaction may occur in the preparation, especially active group reaction with the API, thus affecting the production quality of the product, the stability and curative effect of the drug. Therefore, the selection of pharmaceutical excipients is of great importance in dosage form design and preparation production. The selection of excipients can be comprehensively considered from the following aspects:
1. First of all, the appropriate preparation process and excipients must be selected based on the physical and chemical properties of the drug itself, such as its solubility, fluidity, polycrystalline type, sensitivity to humidity, heat, light, pH, and stability in vitro and in vivo. For example, if the drug is sensitive to wet and heat, it can only avoid the wet process and choose the powder direct pressure or dry grain process. If these two processes require high material liquidity, some accessories with good liquidity, such as T80 lactose / C80 lactose / F100 lactose; 102 / 302 / 702. In addition, by analyzing the structure of the drug itself, we can try to avoid the excipients that can interact with the drug. For example, if the structure of the drug contains ammonia, it is necessary to avoid choosing lactose. Because lactose is a reducing disose, it can have Maillard reaction with the drug containing ammonia. If the solubility of the drug is poor, then in addition to adding the general molding excipients to the tablet prescription, but also pay attention to consider adding a better disintegrant or surfactant.
2. Fully understand the physical and chemical properties of the excipients themselves. It is necessary to have a basic understanding of the function, specification, stability, hygroscopic, fluidity, solubility, pH and viscosity of conventional excipients, so as to reasonably evaluate the influence of physical and chemical properties of excipients on the preparation. For example, hydrophilic excipients are generally: lactose, mannitol, polyethylene glycol, povidone, etc. If it is a hydrophobic drug, it is necessary to add these hydrophilic excipients in the prescription to promote dissolution. For example, hydroxypropanmethyl cellulose (HPMC) has a variety of specifications and viscosity, different concentrations can be used in different dosage forms, so when using, different viscosity and dosage should be selected based on the use.

In the early stage of prescription research, compatibility research on raw materials is generally done, but this work is time-consuming and laborious. As far as possible, some supporting stability data between excipients, or between excipients and raw materials can be obtained, which can save resources and improve efficiency to a certain extent. For example, compounds with amino groups react with lactose, and here are some common types of active functional groups in raw materials.
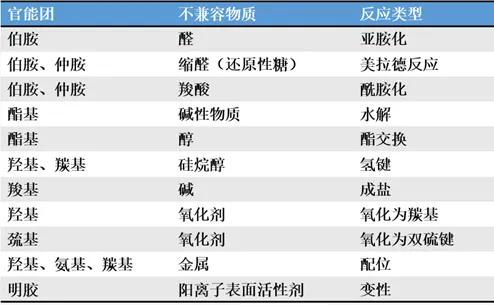
In the early stage of prescription research, compatibility research on raw materials is generally done, but this work is time-consuming and laborious. As far as possible, some supporting stability data between excipients, or between excipients and raw materials can be obtained, which can save resources and improve efficiency to a certain extent. For example, compounds with amino groups react with lactose, and here are some common types of active functional groups in raw materials. The impurities in the excipients that are easy to participate in the reaction mainly include:

3. Select the pharmaceutical excipients based on the dosage form design.
The dosage form design of drugs is a relatively complex process, which needs to be considered based on the physical and chemical properties / biological properties of drugs, clinical treatment and application, feasibility of production and economic cost. Among the basic technical guidelines of chemical drug preparations, the choice of dosage form for unstable drugs in gastric juice; for drugs with hepatic first-pass effect, non-oral administration formulation may be considered; for example, drugs for bleeding, shock and poisoning, usually for acute asthma, and inhalant should be selected.3. Select the pharmaceutical excipients based on the dosage form design. Different dosage forms of pharmaceutical excipients composition is different, such as injection excipients generally include: solvent, solvent, emulsifier, PH regulator, preservatives, complexation, etc., oral liquid system may also need to consider taste, prescription need to add sweeteners, aromatic, natural polymer jelly effective taste agent. The auxiliary materials of oral solid preparations generally include: filler, adhesive, disintegration agent, lubricant, flow aid, etc.
4. Select appropriate excipients based on drug specifications and preparation process.
The specification of drug will affect the choice of excipients, if the drug is high specification preparation, the high specification and the necessary excipients will inevitably lead to the tablet is too large, it may be necessary to select some excipients with superior performance to reduce the dosage of excipients; if the specification is low, the mixing uniformity is the biggest problem, and some liquid and compression excipients can be selected to improve the dispersion uniformity of drugs, different models of lactose can be applied to different preparations. If it is a small size powder direct pressing process, it is suitable to choose improved lactose or complex lactose, like lactose T80 is a block particle obtained by granulation of one water lactose powder, the specification is rough surface, large specific surface area, has certain adsorption effect, conducive to the mixing uniformity of low dose prescription, reduce stratification; and has good mobility and compressibility, its good mobility is also suitable for capsule filling and powder. Lactose C80 is a spray-dried complex, composed of 75% monowater lactose and 25% powder cellulose, which is especially designed for powder direct pressure, combining the filling and adhesion of the two excipients, giving it good mobility and compressibility.


For example, if you choose the capsule dosage form, The selection of the excipients is mainly based on several aspects to consider: first, the compatibility of the excipients and the capsule shell, Since the capsule shell contains a water-soluble protein, Lysine residues are susceptible to react with aldehydes, Without of the capsule, Therefore, we should avoid the selection of auxiliary materials containing aldehyde group; The second is moisture, If the capsule contents are more seriously wet, Will lead to the capsule shell, It is necessary to avoid the selection of excipients with wet absorption; In addition, select the excipients with good mobility based on the dose, If a low dose (<25mg), Hybrid uniformity is the major problem faced, Some excipients with good fluidity should be selected to reduce the risk of uneven mixing.
5. Select appropriate pharmaceutical excipients based on packaging materials. The packaging material includes both the internal packaging and the outer packaging that is in direct contact with the drug. The choice of packaging materials is mainly based on two considerations: not only to provide protection for drugs, but also to consider that packaging materials and drugs should have good compatibility. The commonly used packaging material categories are mainly listed as follows:

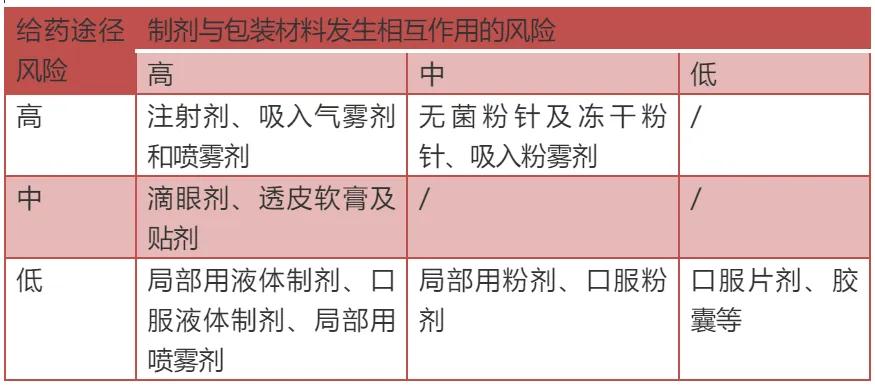
The compatibility studies of different dosage forms and packaging materials are also different. It is necessary to confirm the risk according of the risk of different dosage forms and the risk level of the interaction between the preparation and packaging. Compared with ordinary oral solid preparations, inhaled aerosol or spray, injection, eye solution, etc., after direct contact with the human tissue or into the blood system, so these types of preparation is considered a high risk level of varieties, these varieties and packaging material interaction risk is higher, need to do full compatibility research. In addition, most of the liquid preparation in the prescription contains functional excipients such as suspension, preservatives, antioxidant, these excipients may promote the dissolution of the ingredients in the packaging material, so the possibility of interaction with packaging material is larger, need based on the results of the packaging material compatibility, to choose more stable, does not interact with the packaging material excipients.
To sum up, in addition to the above several items, the selection of pharmaceutical excipients may be based on the market pricing and policy of drugs, from the perspective of economic cost, so as to produce the best quality products at the lowest cost.
reference documentation:
[1] Basic technical guidelines for the research of chemical and pharmaceutical preparations
[2] German beauty pharmaceutical lactose
[3] Hou Huimin, application technology of pharmaceutical excipients, second edition, Beijing: China Medical Science and Technology Press, 2002
[4] Liu Haibo, a study on pharmaceutical excipients [J]. Friends of Biochemical and Pharmaceutical and Chemical Industry, 2006
[5] Liu Yan, study on the compatibility of drugs and packaging materials, Tianjin Drug Packaging Material Testing Center
The companys product recommendation:
1.117391-50-1 https://www.bicbiotech.com/product_detail.php?id=5415
2.5678-47-7 https://www.bicbiotech.com/product_detail.php?id=5416
3.129042-57-5 https://www.bicbiotech.com/product_detail.php?id=5417
4.151911-23-8 https://www.bicbiotech.com/product_detail.php?id=5418
5.68208-21-9 https://www.bicbiotech.com/product_detail.php?id=5419




