On March 9, Novo Norde's weight loss therapy Wegovy (semaglutide, Seglutide) was approved by the FDA for a new indication. Smesiglutide became the first weight loss drug approved to assist adults with cardiovascular disease and obesity or overweight to prevent major cardiovascular events.
In November 2023, Novo Nordisk published the impact of simegallutide on cardiovascular health and published the results of the phase III SELECT trial.2.4 mg Wegovy on standard therapy caused a 20% reduction in major cardiovascular adverse events compared to placebo.
"Wegovy is now the first weight-loss drug approved to help adults with cardiovascular disease, obesity, or overweight prevent life-threatening cardiovascular events at a high risk of cardiovascular death, heart attack and stroke," said John Sharretts, director of the FDA's Diabetes, Blood Disorders and Obesity division. Providing a treatment option that proves to reduce this cardiovascular risk is a major advance in public health ".
Listing and sales situation
Smesiglutide injection for type 2 diabetes and weight loss were approved by FDA in 2017 and 2021 under the trade names Ozempic and Wegovy, respectively. Since weight loss, simegallutide has been expanding, and good news has been reported in clinical studies on heart disease, stroke, and chronic kidney disease. At present, the treatment of sigroglutide for NASH, Alzheimer's disease, heart failure, peripheral artery disease and other indications have entered the phase 3 clinical stage, the prospect is worth looking forward to.
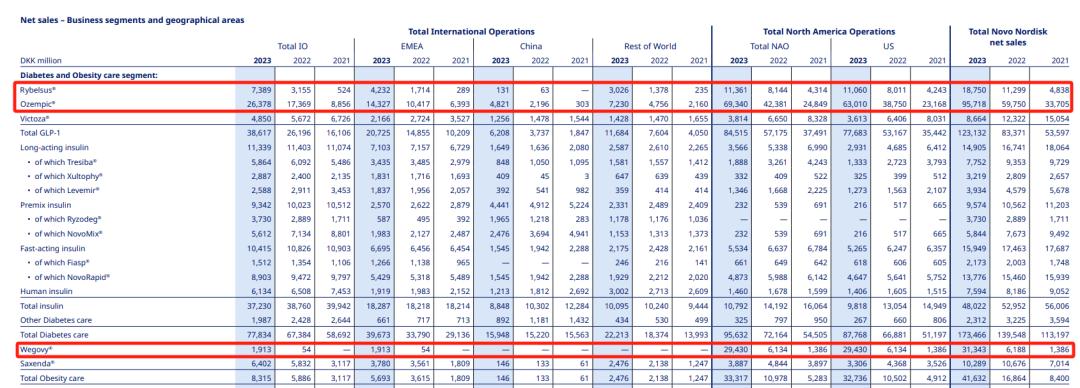
Novo Nordisk reported that profits surged in 2023 due to strong sales of its GLP-1 drug. In 2023, the total sales of siglutide has reached 145.811 billion, about 21.2 billion US dollars, making it the largest "money printing machine" of the company. Among them, Rybelsus (oral simegallutide) sold up to 18.75 billion Danish kroner ($2.721 billion) in 2023, Ozempic (simegallutide) sold 95.718 billion Danish kroner ($13.892 billion), and Wegovy (simegallutide) sold 31.343 billion Danish kroner ($4.549 billion).
 X
X- sum up
- At present, novo nord for GLP-1 class drugs are developing chronic kidney disease, nonalcoholic steatohepatitis, Alzheimer's disease and other chronic disease indications, the current megallutide treatment of nonalcoholic steatohepatitis, Alzheimer's disease, ejection fraction of heart failure (HFpEF), peripheral artery disease indications have entered the phase 3 clinical stage. With the positive results of GLP-1 drugs in a number of preliminary clinical studies and the further deepening of related basic research, GLP-1 drugs are expected to provide effective treatment to more people with indications in the future.




