In the early hours of March 15, Resmetirom, a biomedical company Madrigal Pharmaceuticals, was approved by the FDA to treat adult patients with metabolic dysfunction-related steatohepatitis (MASH, formerly known as NASH), the world's first approved MASH drug.
Resmetirom Is an oral selective agonist of the thyroid hormone receptor β (THR- β) that targets the liver. In MASH, thyroid hormone β activity is impaired in the liver, leading to reduced mitochondrial function and β -oxidation of fatty acids, subsequently leading to inflammation and liver fibrosis, and Resmetirom aims to target this underlying etiology.

Resmetirom Mechanism of action of drugs, source: company official website
In December 2023, Madrigal had announced that the phase III MAESTRO study of Resmetirom for MASH had reached a dual primary endpoint. Phase III clinical trial data show that compared to placebo group, patients taking two doses of Resmetirom drug reached two endpoints: patients have a higher level of symptom relief and liver fibrosis improvement, reached the main endpoint of non-alcoholic fatty liver disease activity score (NAS), but also achieved the low density lipoprotein cholesterol level (LDL) drop, reached the key secondary endpoint
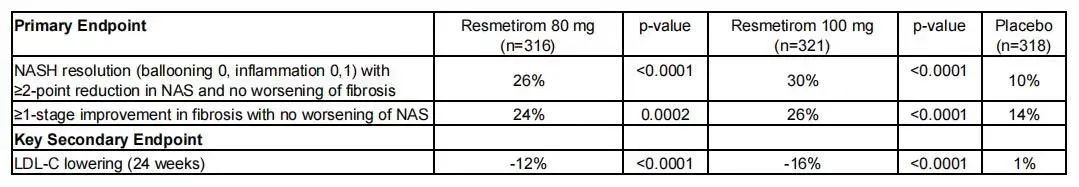
Efficacy analysis of the clinical phase trial, source: the company's official website
In addition, study data based on the MAESTRO-NAFLD-1 clinical trial showed that Resmetirom was safe and well tolerated, and had statistically significant improvements in key indicators of liver and cardiovascular health.
Blue ocean market for MASH drugs
MASH is a severe type of metabolic dysfunction-related fatty liver disease (MAFLD) and is defined as more than 5% of hepatic steatosis, combined with inflammation, hepatocellular injury, with or without fibrosis.
The occurrence of MASH may be the result of the interaction of environmental, genetic, dietary, and metabolic factors, whose histopathological changes include fatty acid accumulation, mitochondrial dysfunction, free radical production, oxygen stress, lipid peroxidation, and endotoxin-mediated cytokine release.
Globally, there are now more than 100 million MASH patients, and 22 million MASH people in the United States have MASH alone, with 8 million people having MASH with severe liver fibrosis.
Since chronic inflammation and liver fibrosis in the MASH stage will cause irreversible damage to the patient liver, it is considered to be a critical stage for progression to end-stage liver disease such as cirrhosis, liver failure or hepatocellular carcinoma.
Due to the complex pathogenesis, since the discovery of MASH was found in 1980, there has been no MASH treatment approved by the US FDA and the European EMA, and Gilead, Novartis, Pfizer, Intercept, Genfit and other pharmaceutical companies have failed here.
The MASH market demand is extremely large. According to Frost Sullivan, the global MASH drug market has reached $1.9 billion in 2020 and will reach $32.2 billion in 2030, with a compound annual growth rate of 24.6%. China's MASH drug market will reach RMB 700 million in 2020 and is expected to reach RMB 35.5 billion by 2030, with a compound annual growth rate of 61.4%.
In order to encourage the development of new drugs for MASH, the Global Liver Association announced in 2023 that it would change it from non-alcoholic steatohepatitis (NASH, Nonalcoholic Steatohepatitis) to metabolic dysfunction-related steatohepatitis (MASH, Metabolic dysfunction-associated steatohepatitis), calling for recognition of the importance of precision therapy and patient-centered care in the treatment of MASH.
Now, with Resmetirom becoming the first MASH drug approved by the FDA, more than 40 years of victory is come.
Of course, in addition to Madrigal, there is no lack of domestic and foreign drug companies targeting the MASH market. According to incomplete statistics, there are more than 500 MASH new drug projects under development in the world, of which more than 200 projects are in the clinical development stage.




