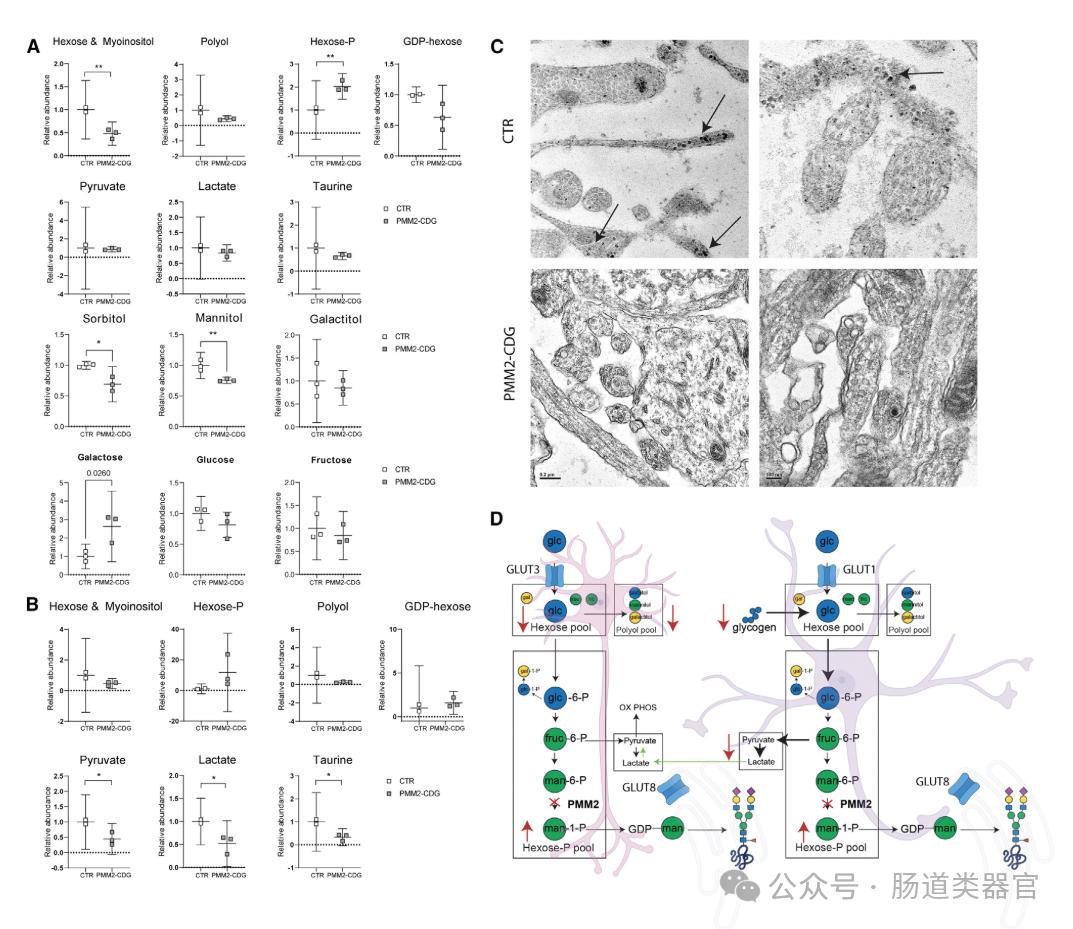
In recent years, a rare genetic metabolic disorder called phosphate mannosyltransferase 2 congenital glycosylation defect (PMM 2-CDG) has attracted the attention of the scientific community. PMM 2-CDG mainly presents with neurological symptoms, but the specific brain-related changes are not fully understood. New studies use human induced pluripotent stem cells (hiPSC) -derived nerve cells and cerebral cortical organoids to reveal abnormalities in specific brain mechanisms in patients with PMM 2 deficiency. The study found that 2D neural network activity was abnormal in PMM 2-CDG patients, and 3D human cortical organoids (hCOs) showed reduced protein glycosylation, impaired mitochondrial structure, and abnormal glucose metabolism, revealing the interference in energy metabolism. These findings improve our understanding of the relationship between PMM 2-CDG and brain-related perturbations, and provide a new direction for future therapeutic interventions.
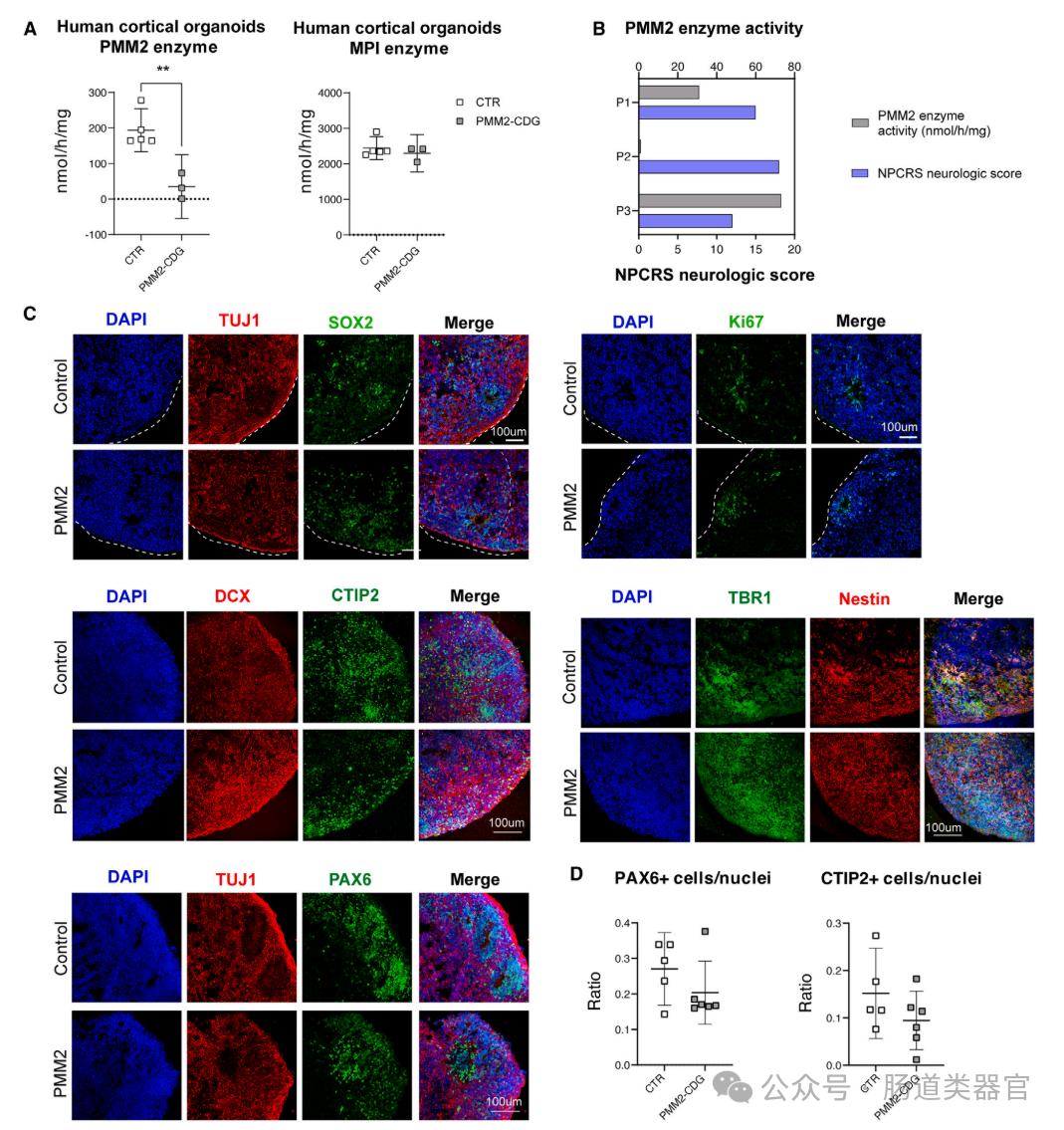
Human cerebral cortical organoids (hCOs) were generated using 3-dimensional human induced pluripotent stem cells (hiPSCs) to study the effect of PMM 2 deficiency on neural maturation. This three-dimensional organoid model has several significant advantages over traditional iNeurons (i. e. neurons directly differentiated from hiPSCs): they do not bypass the early neural progenitor stage, can be cultured for long time, allow the study of multiple cell types, and can be easily tested by different omics techniques. The PMM 2 enzyme activity was first assessed in PMM 2-deficient hCOs and compared with the control group (CTR). The results showed a significant reduction in PMM 2 enzyme activity in PMM 2-deficient hCOs. Furthermore, we examined the associations between PMM 2 enzyme activity of hCOs and the neurological scores of individuals derived from these organoids and found that improved PMM 2 enzyme activity was associated with lower neurological scores.
Furthermore, studies explored whether global differentiation or maturation of PMM 2-deficient hCOs was affected, assessing a range of neural differentiation and maturation markers by immunohistochemistry (IHC). Results did not observe any significant size or structural defects, or differences in the abundance or distribution of markers of neural stem cells (NESTIN, PAX 6, and SOX 2), neurons (doublecortin [DCX], TBR 1, CTIP2, and TUJ 1), or proliferation (Ki67) markers.
Finally, the transcriptome consequences of PMM 2 deficiency in hCOs were determined by RNA sequencing of organoids maintained at different time points (DIV 95, DIV120, DIV170). These time points are intended to include the developmental phase before and after the gliogenesis switch. At the transcriptomic level, hCOs of different genotypes (e. g., PMM 2-CDG hCOs and CTR organoids) showed significant clustering at all time points, indicating that the effects of PMM 2 deficiency are not transient and will persist during development.