On March 18, the FDA approved Lenmeldy, a gene therapy developed by Orchard Therapeutics, to treat children with abnormal leukodystrophy (MLD). It is reportedly the first gene therapy for MLD approved in the US, and the US pricing has not been announced.
MLD is a rare and fatal genetic disorder in which the ARSA mutation (22q13.33 q) causes it to metabolize thionormally, causing damage to the central and peripheral nervous systems. Children will eventually deteriorate into a vegetative state, which may require 24h of intensive care, and most patients will die within five years of their illness, bringing a huge financial burden to their families.
About one in every 40,000 people in the country.
Orchard Is the core of autologous stem cells in vitro modification after gene therapy, the gene therapy is mainly divided into three steps: first, from patients with rare disease in the gene mutation extract hematopoietic stem cells (usually bone marrow), and then in vitro to stem cells genetic modification (usually lentiviral vector carrying normal genes), finally will be modified autologous stem cell transplantation back to the body. The biggest advantage of this therapy is that the donor and recipient of the stem cell are the same person, which avoids the immune rejection caused by the third party donor and eliminates the occurrence of complications.
Lenmeldy (atidarsagene autotemcel, formerly known as OTL-200) was developed using this technology to use lentiviral vectors to introduce the ARSA transgene encoding arylsulfatase-A into autologous CD34 positive hematopoietic stem cells and progenitor cells of MLD patients, so as to restore arylsulfatase-A expression by one-time treatment, prevent or slow disease progression.
Libmeldy After nearly 20 years of research and development, in the first human test in 2010, in December 2020 the first approval in Europe (trade name: Libmeldy), and in February 2022, with the national health service agreement, allowing Libmeldy applies to England and Wales within the scope of European licensing MLD children, it is reported price of 2.8 million pounds (about $3.56 million, at the latest exchange rate 1 $1.2724).
This FDA approval, based on data from 37 children with 12 years of follow-up (median 6.76 years), showed a significantly longer overall survival and a significant reduction in motor impairment or death in patients treated with Lenmeldy compared to untreated children. For safety, Lenmeldy showed good tolerability with no treatment-related serious adverse events or death.
Last year, Kyowa Kirin prepaid $387.4 million for Orchard and acquired the company's gene therapy, then still code-named OTL-200. Now, Lenmeldy's approval also marks a Kyowa Kirin investment victory.
About the Orchard Therapeutics
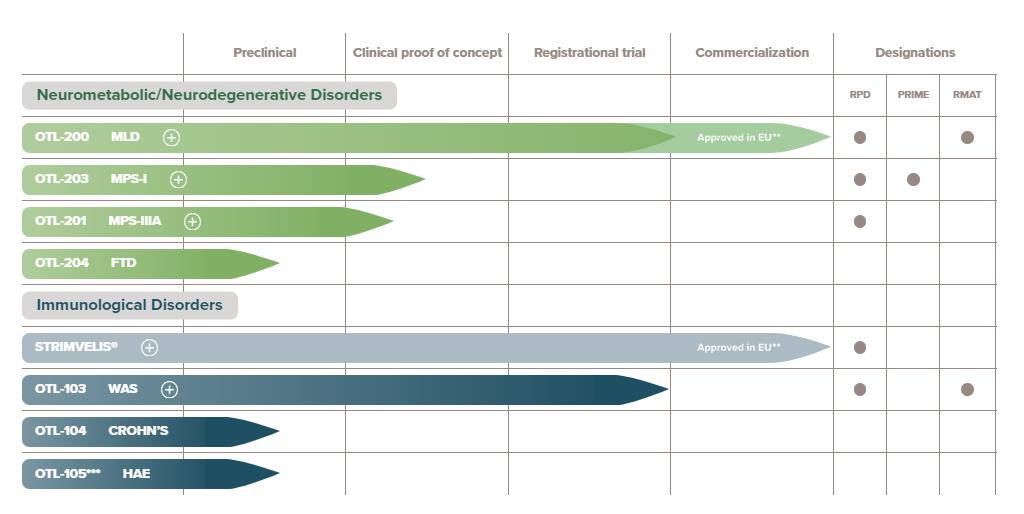
Orchard Therapeutics Founded in September 2015, it is a biotechnology company that uses genetically modified T cells to treat rare diseases. Orc hard The main research targets are severe combined immunodeficiency disease (ADA-SCID), Wiskott-Aldrich syndrome (WAS), X-linked chronic granulomatosis (X-CGD), heteroected leukodystrophy (MLD), mucopolysaccharidosis A (MPS-subA), mucopolysaccharidosis subtype B (MPS-subB) and B thalassemia. Among them, Strimvelis, a gene drug for treating ADA-SCID, was approved by EMA in 2016.
Research and development pipeline