On August 27,2025, the U.S. Food and Drug Administration (FDA) issued a nationwide voluntary recall notice for Unichem Pharmaceuticals (USA) Inc., regarding a batch of Cyclobenzaprine Hydrochloride Tablets (USP 10 mg) produced by the company due to labeling errors. Specifically, vials that should have carried the 10 mg Cyclobenzaprine label were mistakenly labeled with the 7.5 mg Meloxicam Tablets (USP 7.5 mg) information. While this error appears minor, it may pose serious health risks, particularly for specific patient populations, making the potential threat particularly concerning.
Pharmaceutical recalls are typically classified into multiple tiers, with this particular case falling under the "consumer-level" category. This indicates that the products have already reached end consumers, necessitating immediate public awareness campaigns and swift action. Unichem Corporation announced the recall through the FDA's official notification system, emphasizing that it was a voluntary initiative supported by the U.S. Food and Drug Administration (FDA). Despite no adverse event reports related to this batch of products having been received so far, the company has taken decisive action to prevent potential health risks.
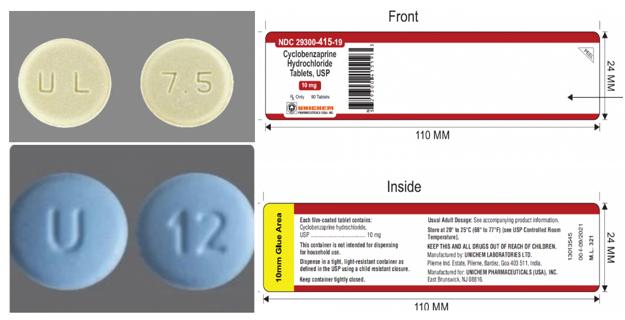
So why has the labeling error caused such significant concern? The key lies in the substantial differences between the pharmacological actions, indications, and target populations of the two medications. Meloxicam, a nonsteroidal anti-inflammatory drug (NSAID), is commonly used to treat osteoarthritis, rheumatoid arthritis, and juvenile rheumatoid arthritis. Its common side effects include cardiovascular events, gastrointestinal bleeding, kidney impairment, severe allergic reactions, and skin reactions. Particularly for patients concurrently taking other NSAIDs or anticoagulants, those allergic to meloxicam, or individuals with pre-existing conditions, accidental ingestion may lead to serious consequences. In contrast, cinnarizine is a muscle relaxant typically used as an adjunct therapy to relieve spasms caused by acute musculoskeletal pain. The two drugs also differ markedly in appearance: Meloxicam 7.5mg tablets appear as light yellow, flat oblong tablets with "UL" and "7.5" markings; while cinnarizine 10mg tablets are blue film-coated convex pills marked "U" and "12". However, once labeling errors occur, patients find it difficult to distinguish the medications through visual inspection alone.
The product being recalled has batch number GMML24026A, with an expiration date of September 2027 and NDC code 29300-415-19. It is packaged in 90-tablet bottles. The product has been distributed nationwide through distribution channels and may have already reached pharmacies or even patients. Unichem has engaged third-party recall service provider Inmar to assist with return processing. The company has urged downstream distributors, retailers, and pharmacies to immediately stop selling this batch of products and notify consumers who may have purchased the medication to return their orders.
While medication label errors are uncommon, they often indicate potential flaws in pharmaceutical manufacturing and quality control systems. The production process requires rigorous oversight at every stage—from label printing and packaging to quality inspection. Any oversight—whether due to human error, equipment calibration mistakes, or insufficient quality checks—can lead to such incidents. These issues not only endanger patient safety but also severely damage a company's reputation and legal standing. This is precisely why global regulatory agencies enforce exceptionally strict regulations on drug production and labeling procedures.
For consumers, this incident serves as a stark reminder to double-check the appearance, label information, and doctor's instructions before taking any medication. If in doubt, consult a pharmacist or physician immediately. Meanwhile, patients and healthcare professionals should proactively report any suspected adverse reactions or product quality issues.
Pharmaceutical safety forms the bedrock of public health systems, where even a seemingly minor labeling error can escalate into a widespread health crisis. Pharmaceutical manufacturers must continuously refine quality management systems, enhance staff training programs, implement automation and smart technologies to minimize human errors, while establishing efficient and transparent recall mechanisms to mitigate risks. Regulatory authorities should strengthen inspections of production facilities to elevate industry-wide standards. Industry stakeholders should learn from each incident to drive improvements in drug safety protocols and operational practices. Only through coordinated efforts among enterprises, regulators, and the public can we build a more reliable and secure environment for pharmaceutical use.
Source: FDA website
Disclaimer: This article is only for the purpose of knowledge exchange and sharing, and does not involve commercial publicity, and does not serve as relevant medical guidance or medication advice. If there is any infringement, please contact us for deletion.
Our products are recommended:
1.1423715-37-0 https://www.bicbiotech.com/product_detail.php?id=6327
2.127199-13-7 https://www.bicbiotech.com/product_detail.php?id=6328
3.37052-78-0 https://www.bicbiotech.com/product_detail.php?id=6329
4.200933-27-3 https://www.bicbiotech.com/product_detail.php?id=6330
5.405911-17-3 https://www.bicbiotech.com/product_detail.php?id=6331




