On March 14,2024, the U. S. Food and Drug Administration (FDA) announced the approval of Madrigal Pharmaceuticals's leading Rezdiffra for the treatment of patients with non-alcoholic steatohepatitis (NASH, also known as MASH) with advanced liver fibrosis. NASH with advanced liver fibrosis has not been approved by the FDA before, and Rezdiffra's approval is landmark as the first and only FDA approved drug for NASH.
graph 1Rezdiffra
Non-alcoholic steatohepatitis (NASH) is a late form of alcoholic fatty liver disease (NAFLD), the global prevalence of about 3% ~ 5%, NASH is the main cause of liver-related death, is the global health system growing burden, according to the global liver institute data, is expected by 2030, the world will have 357 million people life affected by NASH. Once a patient develops NASH in intermediate to advanced liver fibrosis (eligible for stage F2 to F3), the risk of liver-related death and other liver-related events (such as transplantation) also increases significantly. Patients with significant liver fibrosis have an approximately 10 – 17-fold increased risk of liver-related death as compared to patients without liver fibrosis. NASH is rapidly becoming the leading cause of liver transplantation in the USA. Patients with NASH are at increased risk of heart attack, stroke and death, and NASH has become the main cause of liver cirrhosis and hepatocellular carcinoma in European and American countries. Before that, there was no effective drug treatment method.
In patients with NASH, the thyroid hormone receptor- β (THR- β) function in the liver is impaired, leading to decreased mitochondrial function and β -oxidation of fatty acids, and increased fibrosis. Rezdiffra Is a selective agonist targeting the hepatic thyroid hormone receptor- β (THR- β) whose active component is Resmetirom, yielding a maximum response of 83.8% compared to triiodothyronine (T3) and an EC50 of 0.21 µ M in the in vitro THR- β activation function assay. The same thyroid hormone receptor α (THR- α) agonism function test showed a resmetirom efficacy of 48.6% versus T3 and an EC50 of 3.74 µ M. THR- β is the main form of THR in the liver, and stimulation of THR- β in the liver reduces intrahepatic triglycerides, while the thyroid hormone action outside the liver (including heart and bone) is mainly mediated through THR- α.
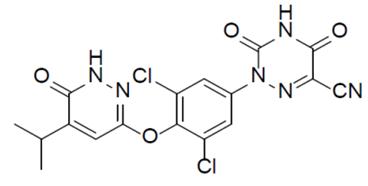
Figure Figure 2 resmetirom Structure
Previously, drug therapy for NASH has not been approved. Given an unmet need for NASH, the FDA developed an accelerated approval approach that allows conditional approval based on the implementation of either of the two histological endpoints (improvement of liver fibrosis stage or remission of NASH) that are considered likely to predict clinical benefit, including death from any cause, liver transplantation, or decompensation.
The accelerated approval of the Rezdiffra is based on the results of a phase III MAESTRO-NASH clinical trial recently published in the New England Journal of Medicine. This clinical trial was used to evaluate the efficacy and safety of MGL-3196 in NASH and fibrosis, enrolling 1759 NASH patients. After 52 weeks of treatment, 100 mg and 80 mg Rezdiffra showed statistically significant improvement in both primary endpoints: NASH remission with no further fibrosis, and the proportion of patients with at least one phase of fibrosis improvement versus placebo, and no NASH deterioration. In addition, the key secondary endpoint of low-density lipoprotein content (LDL-C) was significantly lower compared to the placebo group. Rezdiffra Can improve liver fibrosis and relieve NASH in NASH patients with middle and advanced liver fibrosis.
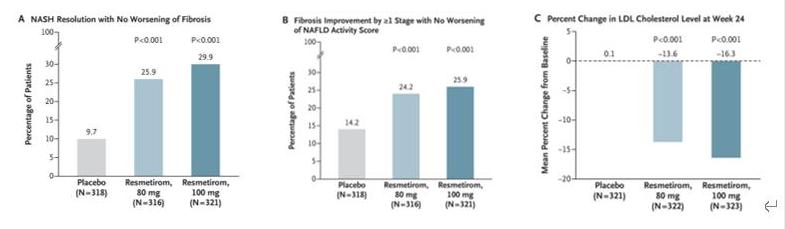
Figure 3 Trial results for the primary and key secondary endpoints
From 2012 American heart association scientific annual meeting (AHA2012), Madrigal released the company's core products MGL-3196 phase I clinical data, Rezdiffra experienced more than ten years of clinical research and development, a comprehensive evaluation of the Rezdiffra safety, effectiveness, pharmacokinetics and drug interaction research, the following figure combed the Rezdiffra in the decades of clinical trials and the test state.




