Alzheimer's disease is not a simple linear disorder driven by a single molecular abnormality, but rather a systemic neurodegenerative process that evolves over time. The abnormal deposition of amyloid protein, excessive phosphorylation of tau protein, dysregulated neuroinflammation, synaptic dysfunction, and disruption of brain homeostasis interweave to form a highly complex pathological network with mutually amplifying effects. Over the past two decades, research has repeatedly demonstrated that interventions targeting a single pathway or a single terminal pathological marker often fail to achieve stable and reproducible clinical efficacy. Against this backdrop, therapeutic strategies that can modulate the production of pathogenic proteins at a more upstream level during the early stages of the disease have regained attention in both academia and industry. Small nucleic acid drugs, particularly siRNAs, provide a technical alternative to antibodies and small-molecule drugs by directly interfering with mRNA levels to inhibit protein translation, offering a novel approach to intervening in neurodegenerative diseases at their "source." Alnylam Pharmaceuticals is the most representative company in this technological pathway. As a pioneer in the industrialization of RNAi drugs, Alnylam has launched multiple products in the fields of rare diseases and cardiovascular metabolism, accumulating systematic technical advantages in delivery chemistry, stability modification, and sustained drug efficacy control. In recent years, with the gradual maturation of central nervous system delivery technologies, Alnylam has extended its RNAi platform to the field of neurodegenerative diseases, with Alzheimer's disease being one of its most strategically significant exploration directions.
I. Mechanistic Differences Between Small Nucleic Acid Drugs and Traditional AD Drugs
1. Mechanism of RNAi
Traditional small-molecule drugs and monoclonal antibodies primarily act at the protein level, typically inhibiting the function of pathogenic proteins, blocking signal transduction, or neutralizing their activity by directly binding to them, thereby alleviating disease symptoms. The fundamental limitation of this mechanism lies in its ability to target only synthesized proteins without preventing the continued production of new pathogenic proteins. If pathogenic proteins are likened to continuously growing weeds, traditional drugs resemble the above-ground parts or neutralize the toxicity without addressing the root cause, often necessitating long-term, repeated administration with limited sustained efficacy. In contrast, small nucleic acid drugs (such as siRNA) exhibit a distinct mechanism of action: they do not directly target proteins but instead utilize the innate RNA interference mechanism within cells to precisely target mRNA—the "template" for protein synthesis. Upon entering cells, siRNA is loaded into the RNA-induced silencing complex (RISC) and degrades mRNA through enzymatic degradation, thereby blocking protein synthesis before it can occur. This mechanism endows small nucleic acid drugs with two inherent advantages:
1.The intervention site is positioned more upstream, theoretically enabling high-proportion knockdown of the pathogenic protein.
2.The compound exhibits prolonged pharmacological effects. Due to the catalytic properties of the RISC complex and the chemical modification technology of Alnylam, it can achieve sustained target inhibition for several months or even up to one year.
For Alzheimer's disease, which has a long course and requires long-term intervention, this low-frequency, long-term, and stable intervention model is theoretically highly attractive.
2. Alnylam's Technological Moat: Delivery and Chemical Modification
The primary obstacle to the industrialization of RNAi drugs is not the mechanism of action, but rather delivery. Based on this, Alnylam has designed two platforms to overcome this limitation:
1.LNP (lipid nanoparticles): Used for early-stage drugs such as Onpattro, primarily targeting the liver.
2.GalNAc conjugation technology: By linking siRNA to N-acetylgalactosamine, it achieves efficient hepatic delivery via the ASGPR receptor on hepatocyte surfaces.
However, the central nervous system does not express GalNAc-related receptors, and the blood-brain barrier (BBB) further restricts the entry of macromolecular drugs. Therefore, the key to the AD project lies not in the efficacy of RNAi, but in whether siRNA can achieve controlled, widespread, and long-term distribution in the human brain.
II. Central Nervous System Delivery
The central nervous system is highly protected by the blood-brain barrier (BBB), which blocks approximately 98% of small-molecule drugs and nearly all macromolecular drugs from entering brain tissue. For negatively charged, highly hydrophilic siRNA molecules, crossing the BBB is nearly an impossible task. To address this issue, Alnylam developed the C16 conjugation technology, which involves attaching a 16-carbon lipid side chain to a specific site on the siRNA molecule. This modification does not simply enhance hydrophobicity but rather improves the interaction between the molecule and neuronal and glial cell membranes by precisely modulating its physicochemical properties, thereby enhancing intracellular uptake efficiency. Preclinical studies have demonstrated that after intrathecal injection, C16-siRNA can circulate in the cerebrospinal fluid (CSF) and extensively distribute to the spinal cord, brainstem, and deep structures of the cerebral cortex, effectively addressing the uneven distribution of traditional antisense oligonucleotide (ASO) drugs in deep brain tissues. Currently, Alnylam has a clear phased strategy for siRNA delivery in Alzheimer's disease: Phase 1 focuses on intrathecal injection as the primary route. Using ALN-APP as an example, the drug is directly injected into the subarachnoid space via lumbar puncture, allowing it to enter the CSF circulation and bypass the BBB for effective delivery. This approach has been validated in terms of pharmacological efficacy, but invasive procedures and the demand for medical resources pose practical challenges to patient compliance, particularly in cases where long-term prophylactic administration may be required. Phase 2 focuses on exploring non-invasive delivery routes. Alnylam is advancing a transferrin receptor (TfR)-based BBB-crossing delivery strategy by conjugating siRNA with an antibody fragment that binds to TfR on cerebral capillary endothelial cells. This enables active transcytosis through the BBB via receptor-mediated endocytosis, followed by release into the brain parenchyma. Once validated in humans, this strategy will fundamentally transform the clinical application paradigm of CNS RNAi therapeutics.
III. Case Analysis: Molecular Design and Delivery of Mivelsiran (ALN-APP)
Mivelsiran is a systemically chemically optimized siRNA drug, with its core being a double-stranded siRNA sequence targeting the mRNA of the APP (amyloid precursor protein) gene. To address the issue of degradation of naked RNA in vivo, Alnylam adopted an ESC+ chemical modification strategy, introducing 2 '-O-methylation and 2' -fluorination modifications on the ribosomal backbone, thereby significantly enhancing the molecule's tolerance to nucleases and endonucleases and prolonging its in vivo half-life. More critically, the C16 lipophilic ligand conjugated to its 3' end not only alters the physicochemical properties of the siRNA but also directly participates in the delivery process. This modification enhances the interaction between the molecule and the cell membrane, laying the foundation for effective exposure in the central nervous system. In terms of the route of administration, mivelsiran enters the cerebrospinal fluid circulation via intrathecal injection, but the transport from cerebrospinal fluid to brain parenchyma remains a critical limiting step. Here, the role of C16 conjugation becomes evident: it endows the molecule with moderate lipophilicity, enabling it to effectively anchor to the lipid bilayer surfaces of neurons and glial cells in brain tissue, avoiding rapid clearance due to cerebrospinal fluid circulation. Subsequently, the drug enters cells via adsorption-mediated or receptor-mediated endocytosis. Existing preclinical and clinical pharmacokinetic data indicate that this mechanism allows the drug to achieve relatively uniform and sustained distribution in key brain regions such as the hippocampus and cerebral cortex, overcoming the traditional limitation of large-molecule drugs in penetrating deep brain tissues. Unlike antibody drugs that target the clearance of established Aβ plaques, Mivelsiran's intervention mechanism lies in blocking the source. After entering the cytoplasm, the drug is loaded into the RISC complex, which specifically degradesAPP mRNA, thereby inhibiting APP protein translation and simultaneously reducing the production of downstream pathogenic amyloid proteins such as Aβ40 and Aβ42. Data from Phase I clinical trials (NCT05231785) demonstrated that following a single intrathecal dose of 75 mg, the level of sAPPα in the patients' cerebrospinal fluid reached peak inhibition (an average reduction of nearly 70%) within approximately 2 months and remained significantly decreased. The level of Aβ42 declined by about 50% early on. Furthermore, due to the metabolic stability and tissue retention properties of ESC+ chemically modified compounds, the inhibitory effects of sAPPα and sAPPβ persisted for over 6 months and approached 10 months in some dose groups. This confirms that C16-conjugated siRNA achieves long-lasting and potent targeted gene silencing in the human central nervous system.
IV. A Brief Analysis of the Advantages and Disadvantages of Small Nucleic Acid Therapy for Alzheimer's Disease
Can small nucleic acid technologies (such as siRNA) achieve a 'dimension-reducing strike' against traditional therapies in the treatment of Alzheimer's disease (AD), or are they constrained by inherent limitations that hinder breakthrough progress? We can conduct a preliminary analysis using the SWOT framework.
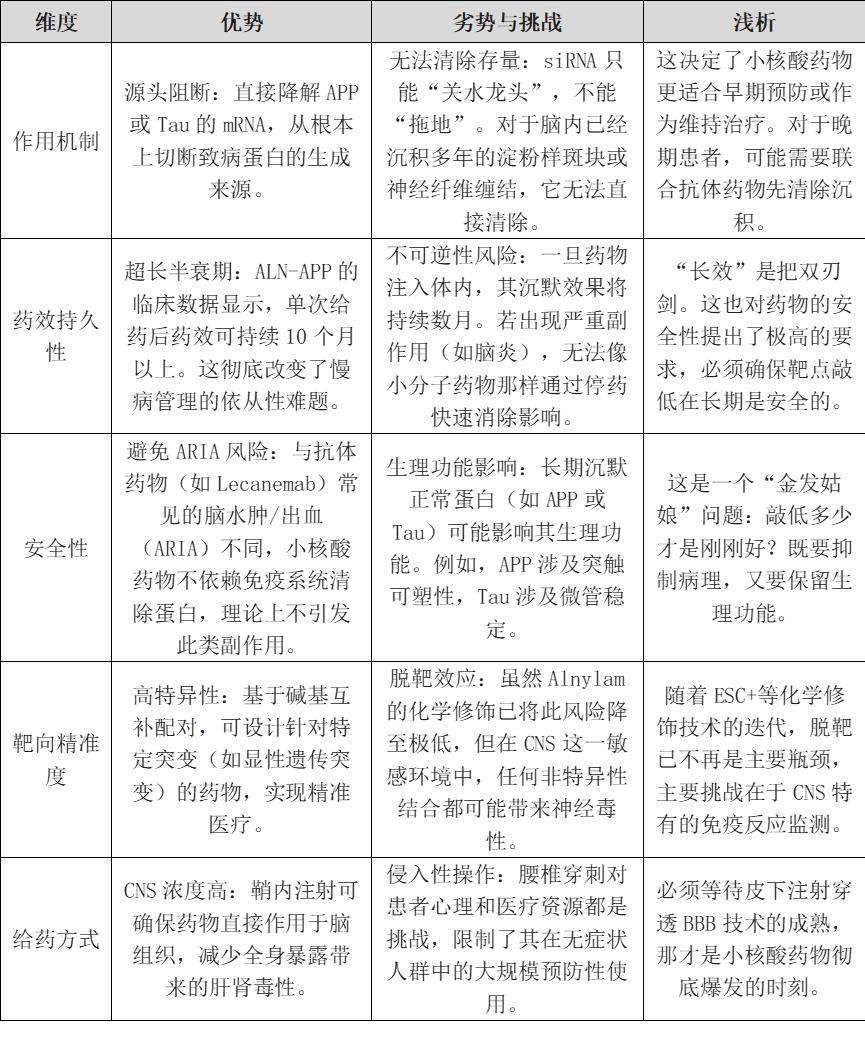
V. Existing Clinical Drugs
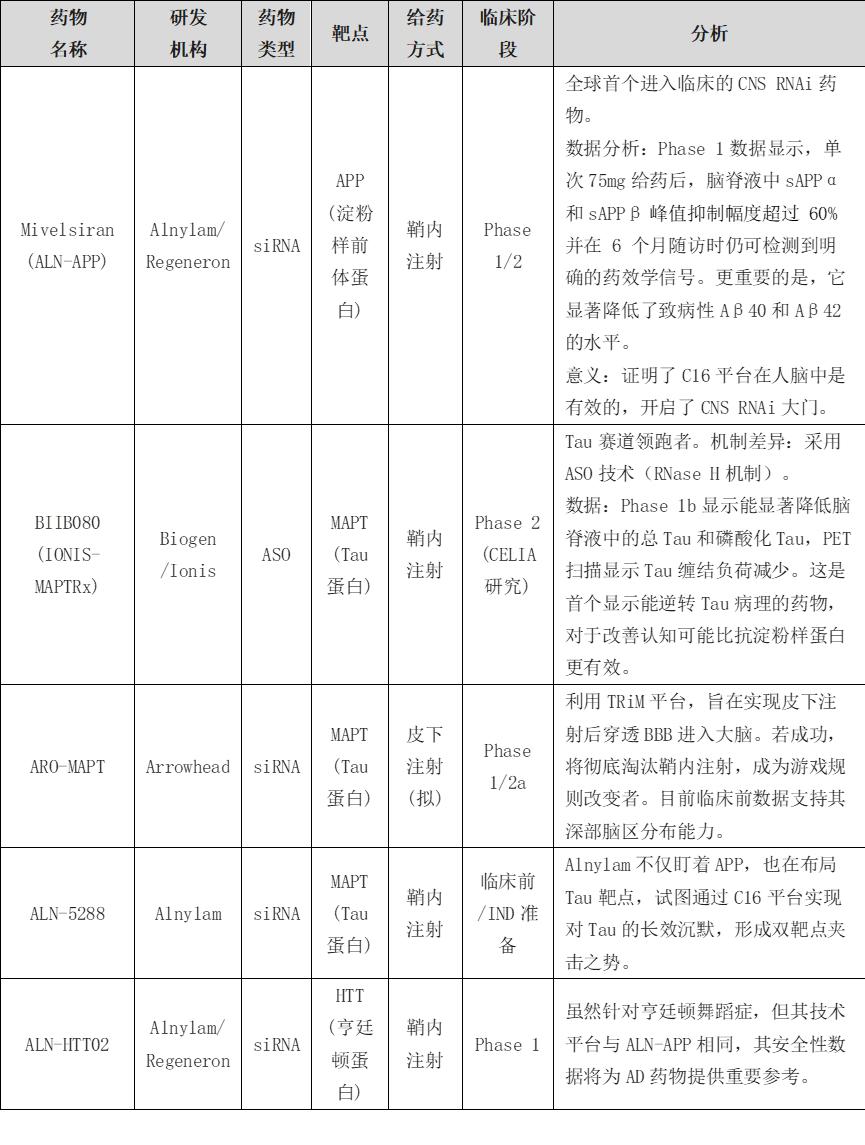
VI. Preliminary Analysis of Potential Therapeutic Strategies for Alzheimer's Disease
Alzheimer's disease (AD) is a complex multifactorial disorder. Over the past decades, drug development targeting single targets (e.g., β-amyloid, Aβ) has repeatedly failed. To achieve a definitive cure rather than merely delaying progression, a phased, multi-target, and multimodal combination therapy strategy is required. Based on the characteristics of Alnylam's siRNA technology and current pathological research evidence, the potential intervention strategies for different stages of AD are briefly analyzed as follows:
1.During the early stage (prodromal or asymptomatic phase), individuals carrying high-risk factors such as APOE4 are identified through genetic screening (e.g., blood tests). When pathological changes (e.g., Aβ deposition) have not yet significantly progressed, long-acting siRNA drugs (e.g., ALN-APP) are administered annually. The mechanism involves siRNA targeting and degrading the mRNA of the APP gene, thereby reducing APP protein expression by approximately 50%. This maintains brain Aβ concentrations below critical aggregation levels, preventing plaque formation. This strategy is highly safe and clinically feasible.
2.During the intermediate stage (mild cognitive impairment phase), a sequential treatment approach is adopted. Initially, monoclonal antibody drugs (such as Lecanemab) are administered for 6-12 months as intensive therapy, which binds to and promotes the immune system to clear accumulated Aβ plaques in the brain. Subsequently, after PET scans confirm that the plaque load has decreased to negative levels, the treatment is switched to long-term ALN-APP maintenance. The mechanism involves the antibody degrading existing Aβ polymers while siRNA continuously inhibits the production of new Aβ, thereby achieving pathological reversal and reducing long-term side effects associated with antibody use (e.g., risk of cerebral edema) and economic costs.
3.In the late stage (dementia phase), multimodal interventions can be implemented, including the introduction of siRNA drugs targeting tau protein (e.g., BIIB080 or Alnylam's ALN-5288) to degrade tau mRNA and block abnormal aggregation and diffusion of tau protein in brain regions, combined with anti-inflammatory drugs targeting microglia (e.g., TREM2 agonists) to inhibit neuroinflammatory activation, and upregulation of neurotrophic factors (e.g., BDNF) via siRNA or other methods to promote neuroprotection. This stage presents the greatest challenges, but multimodal combinations are potentially effective approaches.
VII. Conclusion
Overall, the small nucleic acid (SNA) development practices represented by Alnylam have provided an alternative technical pathway to Alzheimer's disease—a central nervous system disorder long considered' undruggable '—differing from traditional small-molecule and antibody therapies. RNA interference does not aim to directly eliminate existing pathological deposits but rather to achieve long-term, stable source regulation of pathogenic protein production at an earlier molecular level. This approach determines the role of SNA drugs in Alzheimer's disease: they are more likely to serve as critical components of early intervention and long-term maintenance therapy rather than solely bearing the responsibility for late-stage treatment. Alnylam's explorations in central delivery, chemical modification, and pharmacological persistence have overcome the key barriers of siRNA's limited effective distribution and long-term knockdown in the human brain. Meanwhile, practical constraints such as invasive administration methods, safety boundaries of long-term inhibition of physiological proteins, and minimal impact on existing pathological loads clearly delineate the applicability limits of this technical route. Therefore, it is more probable that SNA drugs are not the 'ultimate solution' for Alzheimer's disease but rather an indispensable component of future multi-target, multi-mechanism combination therapy systems.
VIII. References
1.Alnylam Pharmaceuticals. (n.d.). siRNA Delivery Platforms: C16 Conjugates.
2.Alnylam Pharmaceuticals. (2023). Press Release: Delivery to Eye and Lung and CNS.
3.Jadhav, V., & Maier, M. (2024). Emerging Tolerability Profiles of C16-siRNA Conjugates for CNS Delivery. Preprints.
4.Alnylam Pharmaceuticals. (2023). Mivelsiran (ALN-APP) Fact Sheet.
5.Mummery, C., et al. (2023). Tau-targeting Antisense Oligonucleotide BIIB080 in Patients with Mild Alzheimer's Disease: Phase 1b Results. JAMA Neurology.
6.Alnylam Pharmaceuticals. (2023). Interim Phase 1 Results for ALN-APP presented at CTAD.
7.Arrowhead Pharmaceuticals. (2025). Arrowhead Initiates Phase 1/2a Study of ARO-MAPT.
8.Cummings J L, Zhou Y, Lee G, et al. Alzheimer's disease drug development pipeline: 2025[J]. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 2025, 11(2): e70098.
9.Vega M R, Hansen H H, Jensen C S, et al. Transferrin receptor-binding blood-brain barrier shuttle enhances brain delivery and plaque-clearing efficacy of a therapeutic anti-Aβ antibody[J]. Fluids and Barriers of the CNS, 2025, 22(1): 121.
Disclaimer: This article is intended solely for knowledge exchange, sharing, and popular science purposes, and does not constitute commercial promotion, nor should it be regarded as medical guidance or medication advice. For copyright infringement, please contact us for removal.
Our product recommendations:
1.1216124-21-8 https://www.bicbiotech.com/product_detail.php?id=6477
2.1367937-31-2 https://www.bicbiotech.com/product_detail.php?id=6478
3.1216292-78-2 https://www.bicbiotech.com/product_detail.php?id=6479
4.442685-53-2 https://www.bicbiotech.com/product_detail.php?id=6480
5.127956-25-6 https://www.bicbiotech.com/product_detail.php?id=6481




