A acne medication with annual sales exceeding 100 million yuan reflects the ongoing rivalry between the original research company and over a dozen generic drug manufacturers. With Huahai Pharmaceutical's latest entry into the competition, the market landscape of adapalene gel has once again drawn attention. In the current context where "generic drug consistency evaluation" and "volume-based procurement" have become routine practices, competition for such mature products is no longer merely a contest of approval numbers but a comprehensive battle of corporate strategic resolve, cost control, and market penetration capabilities.

Source: CDE official website
market map
The original manufacturer of Adapalene gel is Galderma, a French company, which was first approved for marketing in the United States in May 1996. Leveraging its first-mover advantage and exceptional product quality, it has established a solid foundation in the global market. In China, Galderma has consistently maintained a strong presence in the high-end market, attracting patients with high quality requirements through its brand influence and clinical reputation.
As the patent protection period approaches, numerous domestic pharmaceutical companies have keenly recognized the market potential of adapalene gel and have actively entered the generic drug production arena. Currently, in addition to the original manufacturer, 11 domestic pharmaceutical companies, including Wuhan Nuoyan Pharmaceutical, Fuyuan Pharmaceutical, Wanquan Wantai Pharmaceutical, Jiangsu Fubang Pharmaceutical, and Sichuan Mingxin Pharmaceutical, have obtained production approvals for adapalene gel, forming a competitive landscape of "1 original + 11 domestic." This seemingly vibrant scenario actually conceals underlying complexities.
Among the 11 domestic generic drug manufacturers, only 2 have successfully passed the consistency evaluation. This rigorous assessment evaluates the quality and efficacy of generic drugs, requiring them to meet the same standards as the original research drugs to gain a competitive edge in the market. However, in reality, most generic drug companies face significant challenges during the consistency evaluation process, leading to a phenomenon where there is a surge in applications but a subsequent decline in approval rates.
The root causes can be attributed to the following key factors. From a technical perspective, as a topical formulation, adapalene gel features a complex prescription composition, predominantly in semi-solid form, with characteristics such as multiphase and thermodynamic instability. During the generic drug development process, it is essential to ensure that critical quality attributes—including drug release, absorption, and stability—are consistent with the originator drug, which imposes stringent requirements on pharmaceutical companies' formulation processes and R&D capabilities. Many enterprises encounter challenges in prescription research, process development, and quality studies, resulting in product quality discrepancies from the reference formulation and difficulties in passing the consistency evaluation.
From a market perspective, some pharmaceutical companies may have overestimated their capabilities and market potential during regulatory submissions, failing to adequately assess the challenges and risks involved in drug development. After investing substantial resources in regulatory applications, they often find themselves unable to meet the standards for consistency evaluation, leaving them in a difficult position. Moreover, the intense market competition has driven some companies to rush through the process, compromising quality control during R&D, which further complicates the likelihood of passing regulatory reviews.
In terms of market performance, the original Gao Demei product continues to maintain a dominant position. Leveraging its years of accumulated brand recognition and clinical validation, it achieves high distribution rates and sales volumes across major hospital and pharmacy channels. Particularly in large tertiary hospitals in first-tier cities, the original product enjoys significant trust from both physicians and patients.
The domestic generic drug industry has shown a polarized development trend. A select few companies excelling in product quality and brand promotion have gradually gained prominence in second-and third-tier cities as well as grassroots healthcare markets. By leveraging cost-effectiveness advantages and localized sales strategies, they have secured significant market share. For instance, one domestic pharmaceutical company successfully boosted product awareness and sales through deep partnerships with local pharmacy chains, implementing promotional campaigns and patient education programs.
However, most domestic generic drug manufacturers are at a disadvantage in market competition due to inconsistent product quality and weak brand influence. They often rely solely on low-price strategies to attract customers, resulting in severely compressed profit margins and an unfavorable survival situation.
Why Adapalene?
Adapalene gel has become a competitive hotspot among generic drug manufacturers due to its unique mechanism of action, definitive clinical efficacy, and promising market prospects. As a retinoid non-prescription drug, it holds a pivotal position in the field of acne treatment.
In terms of mechanism, adapalene, as a retinoid compound, primarily reduces the formation of microcomedones by regulating the differentiation of hair follicle epithelial cells, while simultaneously inhibiting the chemotaxis of polymorphonuclear leukocytes to alleviate inflammation. It exhibits both cell differentiation and proliferative activities, achieving dual therapeutic and preventive effects for acne. Clinically, it is indicated for the topical treatment of mild to moderate acne vulgaris (predominantly comedones, papules, and pustules), applicable to the face, chest, and back, and serves as a first-line treatment for acne. Compared to other acne therapeutics, it offers advantages such as definitive efficacy, fewer adverse reactions, and better patient tolerance.
Market data indicate that the performance of adalaparin gel has been remarkably outstanding. In 2024, its sales revenue in the comprehensive hospital market exceeded 100 million yuan, representing a year-on-year growth of 6.36%, demonstrating a sustained upward trend. This achievement is attributed to multiple factors.
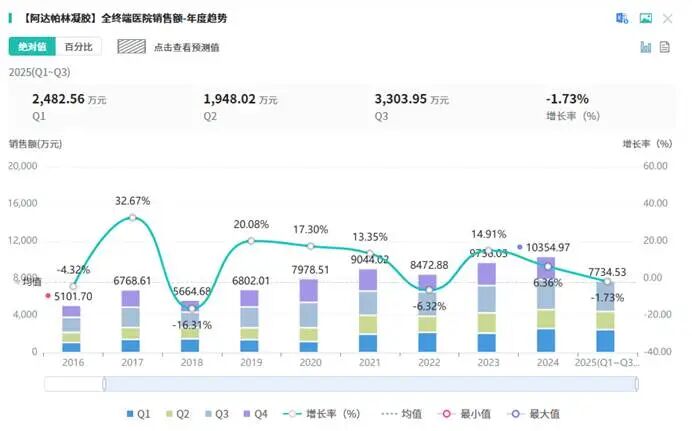
Image source: MoEntropy Pharmaceutical Database
The formulation advantage of adapalene gel is one of the key factors attracting patients and pharmaceutical companies. As a topical gel, it features easy application, rapid absorption, and minimal skin irritation. Compared to oral medications, topical gels can directly act on the affected area, enhancing local drug concentration and therapeutic efficacy while reducing the incidence of systemic adverse reactions. Patients only need to apply the gel to the acne lesions, making the procedure simple and convenient, without the concern of gastrointestinal discomfort associated with oral medications.
Patient adherence is a critical factor influencing the clinical efficacy and market promotion of adapalene gel. As a highly prevalent dermatological condition, acne affects a broad patient population with prolonged disease duration and extended treatment cycles, imposing high demands on medication convenience and tolerability. The topical formulation of adapalene gel allows direct action on affected areas, simplifies administration procedures, and aligns with patients' needs for convenient treatment, thereby enhancing therapeutic compliance. Compared to oral acne medications that require strict adherence to fixed dosing schedules and dosages, adapalene gel reduces the risk of non-adherence behaviors such as missed doses or self-discontinuation, ensuring treatment continuity and efficacy, and ultimately optimizing clinical benefits.
The market appeal of adapalene gel is further enhanced by its substantial existing market base and continuously expanding potential. Epidemiological data indicate that acne is a globally prevalent dermatological condition, with particularly high incidence rates among adolescents. Concurrently, demographic changes and lifestyle shifts have led to a sustained expansion in the patient population affected by acne, providing a stable demand foundation and broad market prospects for adapalene gel. Additionally, with advancements in medical technology and deeper research into the pathogenesis of acne, the clinical applications of adapalene gel have gradually broadened. Beyond its conventional use in treating mild to moderate acne vulgaris, its potential value in managing other inflammatory skin conditions has been preliminarily explored, injecting new momentum into its market growth.
From "First to Copy" to "Efficiency"
With the advancement of the pharmaceutical industry and intensifying market competition, the generic drug market for adapalene gel has undergone profound transformations. Historically, "first-to-market generic drug competition" was the primary strategy adopted by numerous pharmaceutical companies in the adapalene gel market. First-to-market generics often leveraged their early market entry to rapidly capture market share and achieve higher profit margins. This competitive strategy was particularly prominent during the initial phase of adapalene gel market development.
Taking Huahai Pharmaceutical as an example, as a company that has been continuously advancing in the generic drug sector, its strategic layout in the adapalene gel project demonstrates the company's foresight and market insight. To date, Huahai Pharmaceutical has obtained approval and passed evaluation for over 100 drug products, among which 19 are first-in-class products such as lamotrigine tablets, efavirenz tablets, voriconazole tablets, and donepezil hydrochloride tablets. Regarding adapalene gel, on January 12,2026, Zhejiang Huahai Pharmaceutical's marketing application for adapalene gel, filed under Category 4 of the chemical drug registration classification, was accepted. If it can be successfully approved and pass the evaluation, it is expected to become the third domestic pharmaceutical company to pass the evaluation. The entry of Huahai Pharmaceutical has intensified competition in the adapalene gel market and driven transformation across the entire sector.
However, as an increasing number of enterprises join the imitation of adalaparin gel, market competition has gradually entered a deep-water zone. The simple 'first-to-market imitation' strategy can no longer meet the needs of sustainable market development for enterprises. Nowadays, companies place greater emphasis on cost control, channel integration, and brand building. Competition has shifted from mere quantity to continuous optimization of quality and efficiency.
From an industry policy perspective, the implementation of the consistency evaluation policy has profoundly impacted generic drug manufacturers. The policy requires generic drugs to achieve quality and efficacy equivalence with originator drugs, compelling companies to prioritize product quality enhancement during R&D. To pass consistency evaluations, pharmaceutical firms must invest substantial R&D resources, optimize formulation processes, and ensure product quality matches originator drugs. This inevitably increases both R&D costs and time investment. Small-scale pharmaceutical companies, constrained by limited financial and technical capabilities, often struggle to meet consistency evaluation requirements, sometimes even abandoning the project altogether. In contrast, large pharmaceutical companies leverage their robust R&D capabilities and financial advantages to better address consistency evaluation challenges, securing competitive advantages in the market.
In corporate strategy, product line expansion has become a key strategy for pharmaceutical companies competing in the adapalene gel market. Take Huahai Pharmaceutical as an example: By continuously expanding its product portfolio, the company diversifies its offerings and reduces reliance on single products. Beyond adapalene gel, Huahai has also ventured into generic drugs across multiple fields, including antidepressants and anti-HIV medications. This diversified product strategy not only enhances market competitiveness but also mitigates risks. When competition intensifies or unfavorable conditions arise for a specific product, the company can maintain stable performance through sales of other products.
In the context of market competition, product differentiation has become the key to distinguishing enterprises. In the adaloparin gel market, although most products share similar primary ingredients and therapeutic effects, companies can achieve differentiation through innovations in dosage forms, formulations, and packaging. Some enterprises have introduced gel formulations that are easier to apply and absorb, or added other auxiliary components to enhance the efficacy and safety of the product. Simultaneously, companies also strengthen brand building and market promotion to improve product awareness and reputation. By conducting patient education activities and collaborating with medical institutions, they enhance the recognition and trust of physicians and patients in the product, thereby gaining a competitive advantage in the market.
The generic drug market for adapalene gel is undergoing a profound transformation from "first-to-market generics" to "efficiency-driven competition." Companies must adapt to evolving industry policies, adjust their strategies, and focus on enhancing product quality and efficiency. By employing tactics such as product differentiation, cost control, and channel integration, they can stand out in the highly competitive market. In the future, with continuous technological advancements and market expansion, the generic drug market for adapalene gel will maintain its competitive edge, offering patients more high-quality and affordable product options.
References: CDE official website; MoTeng Pharmaceutical Database;
Disclaimer: This article is intended solely for knowledge exchange, sharing, and popular science purposes, and does not constitute commercial promotion, nor should it be regarded as medical guidance or medication advice. For copyright infringement, please contact us for removal.
Our product recommendations:
1.501-94-0 https://www.bicbiotech.com/product_detail.php?id=6482
2.10597-60-1 https://www.bicbiotech.com/product_detail.php?id=6483
3.480-18-2 https://www.bicbiotech.com/product_detail.php?id=6484
4.930-68-7 https://www.bicbiotech.com/product_detail.php?id=6485
5.6859-99-0 https://www.bicbiotech.com/product_detail.php?id=6486




