
On March 19,2024, the US Food and Drug Administration (FDA) announced the approval of Idorsia Pharmaceuticals's new molecular entity, Tryvio (active ingredient: Aprocitentan). Tryvio Is an endothelin receptor antagonist used in the treatment of hypertension that remains not adequately controlled in combination with other antihypertensive drugs. Tryvio Is currently the first and only endothelin receptor antagonist, and is the first approved oral antihypertensive therapy to work through a new treatment route in nearly 30 years.
Global profile of hypertension and commonly used clinical drugs
Hypertension is defined as a high vascular pressure (140 / 90mmHg or higher), and the incidence continues to increase. According to a recent study, the number of people affected by hypertension has almost doubled in the past 40 years, with about 1.13 billion people having hypertension worldwide. The World Health Organization estimates that hypertension causes 7.5 million deaths each year, or about 12.8% of the total global deaths.
Hypertension is usually developed over the years without obvious symptoms. If not treated promptly, it may cause serious complications such as stroke, blindness, heart failure, renal failure, and sexual dysfunction. Clinically, the following 6 types of hypertension treatment drugs are commonly used alone or combined to treat hypertension:
1. Diuretics, targeting the kidneys, help the body drain sodium and water, thus reducing blood volume.
2. A α receptor antagonist, preventing the vasoconstricting effects of norepinephrine or adrenaline, thereby improving blood flow and lowering blood pressure.
3 The β receptor antagonist, blocking the action of adrenaline, makes the heart beat slower, less powerful, and improves blood flow, thus lowering blood pressure.
4. Angiotensin converting enzyme (ACE) inhibitors, stop the formation of angiotensin II, a substance that rows blood vessels and therefore lowers blood pressure.
5. Angiangiotensin II receptor (ARB) antagonist, blocking the action of angiotensin II rather than its formation. Both ACE and ARB inhibitors are usually one of the treatments for patients with chronic kidney disease.
6. Calcium old channel blockers reduce calcium in the heart by blocking calcium into muscle cells. This reduces the contractility of muscle cells and dilates the arteries, thus lowering blood pressure.
Tryvio (Aprocitentan) treatment advantage
It is called refractory hypertension if it is still not reduced by combination therapy with three different classes of antihypertensive drugs (including diuretics). In recent years, despite the development of many new antihypertensive therapies, the last approval of antihypertensive drugs that act through new routes of antihypertensive mechanisms was more than 30 years ago. Tryvio Is the first approved oral anti-refractory hypertension therapy to work through a new therapeutic pathway in over 30 years. Before Tryvio approval, there was no other FDA approved systemic antihypertensive therapy for the ET pathway.
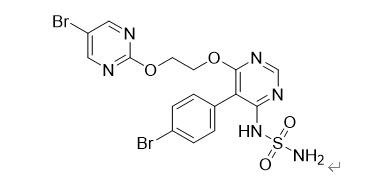
Figure 1 The Aprocitentan Structure
The active component of Tryvio is Aprocitentan, which inhibits the binding of endothelin-1 (ET-1) to endothelin receptor A (ETA) and endothelin receptor B (ETB) receptor. ET-1 and its receptor are highly expressed in vascular endothelium and vascular smooth muscle cells as an important regulator of the hemodynamic dynamic balance. ET-1 is a peptide containing 21 amino acids that can irreversibly bind to its receptor and is currently the strongest and longest lasting vasoconstrictor. In hypertension, ET-1 can also cause endothelial dysfunction, vascular hypertrophy and remodeling, sympathetic activation, and increased aldosterone synthesis. The presence of the interaction between endothelin and endothelin receptor may be responsible for the inability of currently available drugs to control blood pressure.

Figure 2 Endothelin and its endothelin receptor action pathways
FDA approved Tryvio marketing basis
This FDA approval was based on the results of a phase III PRECISION trial of Tryvio analyzing the short-term and long-term efficacy of Tryvio in 730 patients with uncontrolled hypertension in a three-phase study. The first phase is a 4-week double-blind period, Of these, 730 patients were randomized to 12.5mg (n=243), 25mg (n=243) Aprocitentan, or placebo (n=244); The second phase is a single-blind period of 32 weeks (4 to 36 weeks), The patient received 25mg of Aprocitentan (n=704); The third phase is a double-blind withdrawal period of 12 weeks (36-48 weeks), Patients were re-randomized 1:1 to 25mgAprocitentan (n=307) or placebo (n=307).
The results showed that Tryvio reached the primary efficacy endpoint of the trial, four weeks after medication, Tryvio significantly reduced sitting systolic blood pressure (SBP) compared with the placebo group. The Tryvio also reached the key secondary efficacy endpoint of the trial, which was a sustained reduction in patients treated with Aprocitentan versus placebo at weeks 36 to 40, and at week 40 by + 5.8mmHg compared to 25mgAprocitentan.
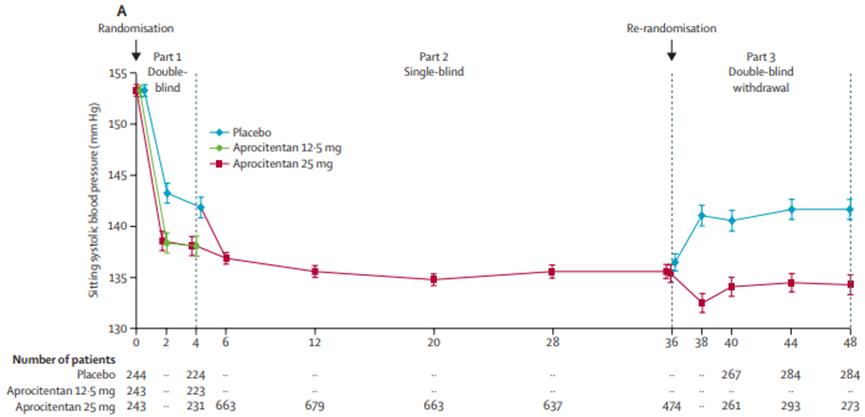
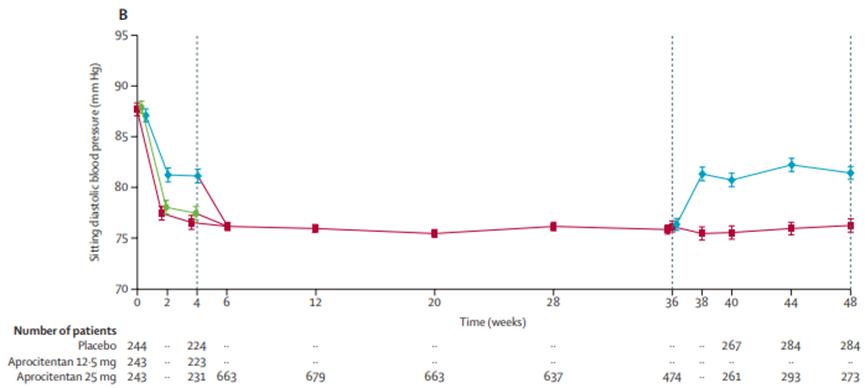
Figure 3 Results of sitting systolic and diastolic blood pressure over time
Tryvio Clinical course
The approval of Tryvio has experienced several years of preclinical and clinical research, completing the safety and efficacy studies of Tryvio across the country, evaluating the TCM generation kinetics and metabolic properties of Tryvio in healthy subjects and patients with liver injury, and evaluating the therapeutic effect and drug interaction studies of Tryvio with other drugs. The positive results of a phase III clinical trial published in the journal The Lancet became the catalyst for the successful marketing of Tryvio. Idorsia submitted its application for the marketing of Tryvio new drugs to the FDA on December 19,2022. After a unified review by the FDA, it was successfully approved by the FDA on March 19,2024. The following figure summarizes the history of Tryvio in different stages of clinical trials.
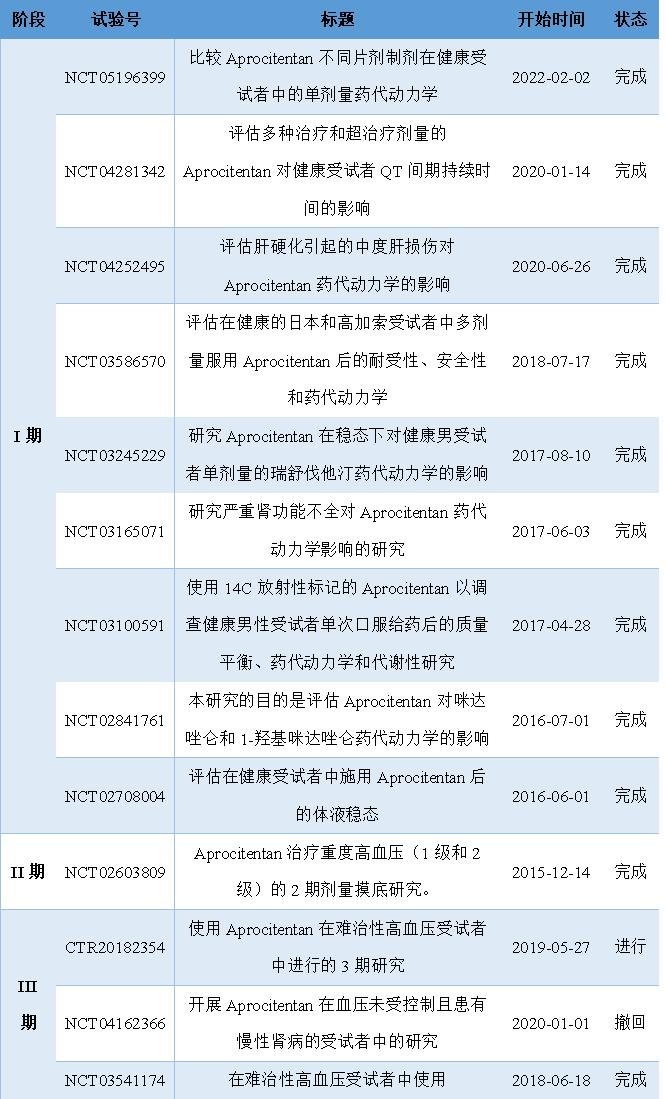
Figure 4 Tryvio Summary of the clinical course
Summarize
Idorsia US President and General Manager Tosh Butt commented, " Tryvio approval in the US is another important milestone for Idorsia. With Tryvio, we obtained an innovative drug with a unique mode of action in the field of systemic hypertension."For many years approved hypertension drugs focus on saline balance regulation, renin-angiotensin-aldosterone system antagonism, reduce extracellular calcium ions into cells, sympathetic activity or nonselective vasodilation, there is not a systemic antihypertensive therapy for endothelin pathway, Tryvio approved to fill the gap, brought new treatment mode for patients with hypertension.
reference material:
[1] FDA official website: https: / / www.fda.gov/
[2]Idorsia Website: https: / / www.idorsia.com/
[3]Schlaich MP, et al.Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial.Lancet.2023 Jan 28;401(10373):268




