When selling pipelines and bodies overseas is the only lifeline, the situation is already very urgent.
On April 3, Danish pharmaceutical company Genmab acquired ADC-Biotech with $1.8 billion. From an optimistic perspective, this means that the ADC track has high prosperity, and China's biotechnology industry has become the birthplace of innovation in the forefront of ADC innovation.
But then he was sad.
On the one hand, CXO, the pioneer of the innovative pharmaceutical industry chain, is forced by decoupling; on the other hand, the core assets of biotechnology are intensively harvested by foreign capital. Since December, four biotech companies have been acquired by overseas drug companies after AstraZeneca bought Genxi Bio for $1.2 billion, Novartis Sinorino Pharma and NuvationBio Baoyuan Pharma.
It is normal for buyers to come from overseas, but none to come from China.
At present, innovative drugs are facing two contradictions: can VC / PE exit the primary market? Biotech Can the research and development cost be recovered by selling drugs?
It is necessary to avoid the distortion of incentive mechanism and the ineffective allocation of innovative resources. BD or merger from overseas should not be the only dawn.
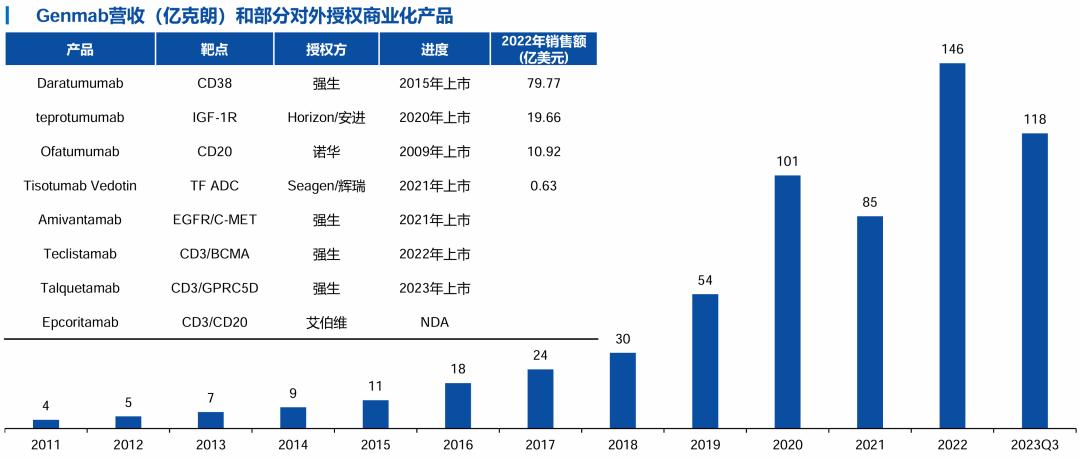
Source: Medical Rubik's Cube database, Wind, Genmab official website, Huachuang Securities
01 I'm too busy
The domestic innovative drug ecosystem is not complete and cannot be metabolized by itself.
Innovative drugs have been verified by data. Among the 316 Biotech companies listed through NASDAQ IPO from 2004 to 2016, only 36 companies can survive for more than 10 years, accounting for 11.4%. The main channel of exit is mergers and acquisitions, delisting and bankruptcy only account for a very low proportion, and China will replicate this path.
But guess the beginning, but can not guess the ending. So far, and the purchase of all from overseas, the domestic big pharmaceutical companies are unable to copy the bottom.
As of 2023Q3 cash reserve of 17.6 billion yuan, Shiyao Group 2023Q4 cash reserve of 13.8 billion yuan, the richest is China biopharmaceutical, 2023Q4 cash reserve of 21.1 billion yuan. In order to cope with the cost control pressure of domestic centralized procurement and medical insurance negotiation, the internal pressure of the deteriorating competition of core products, and maintain the sustainable research and development intensity, there is no spare time to copy the bottom of domestic high-quality Biotech at this stage. For the above 3 BigPharma companies, the r & d expenditure in 2023 will be about 6 billion yuan, 4.8 billion yuan and 4.4 billion yuan, respectively.
Genmab The cash reserve is $4.2 billion (about 30 billion yuan), and the cash cow CD38 mab Darzalex (mab) reached $9.74 billion in 2023, up 22% year on year, and is expected to exceed the $10 billion mark this year. Darzalex Johnson & Johnson is responsible for global commercialization, and Genmab receives 20% royalties.
Genmab It is less well-known in China, but it may become a growth template for China's Biotech.
Genmab The official operation began in February 1999, just two years later than Hengrui Pharmaceutical. The revenue in 2023 is $2.39 billion, equivalent to the revenue of Hengrui Pharmaceutical in the first three quarters of 2023, but the cash reserve is richer, and the annual R & D expenditure is about 30% higher than that of Hengrui Pharmaceutical. Genmab It expects a strong 19% revenue growth in 2024.
Genmab Is a global leader of antibody drugs, is also a pure research and development drug enterprise, all production capacity outsourcing, commercialization and basic external authorization.
In 2008, Genmab acquired PDL BioPharma's 22,000-liter antibody manufacturing plant in Minnesota for $240 million, coinciwith the double blow of severe financial problems and the global financial crisis. At the end of 2009, we decided to sell the manufacturing facilities just acquired from PDL, and buyers were hard to find until $10 million to Baxter (Baxter) in 2013. Since then, Genmab has never purchased manufacturing facilities, instead of outsourcing to meet manufacturing and clinical trial needs.
Genmab Eight antibody products have been approved for marketing, and all commercial cooperation with foreign countries.
At the beginning of its establishment, most Biotech in China had the dream of becoming a comprehensive pharmaceutical enterprise and carrying out the whole chain layout. It is time to wake up, shrink the front line and focus on research and development.
The Fast-follow or Me-better strategy is more efficient and cheaper, which is also one of the reasons why big domestic pharmaceutical companies are reluctant to acquire Biotech. In 2025, the growth history of China's innovative drugs has just reached 10 years, pharmaceutical companies are still weak in research and development and financial resources, and now they can only allow foreign investors to copy the bottom.
02 Jedi born
Genmab Single-handedly created three $1 billion molecules (Darzalex / Tepezza / Kesimpta).
The domestic innovative drug market has not yet nurtured a $1 billion molecule (zebutinib mainly relies on overseas markets), and there is no hope in the short to medium term. Henrui Pharmaceutical PD-1 carilizumab was a flash in 2020, with sales of about 4.89 billion yuan (according to Frost & Sullivan), setting a peak sales record of innovative drugs in China. The Chinese version of Genmab Kangfang biological core product Kadunelimonomab (PD 1 / CTLA 4), 2023H1 sold 605.8 million yuan, and the sales of 2023H2 reached 752 million yuan, but as one of the strongest self-developed new drugs in China so far, it is still far away from $1 billion molecules.
The innovation law of biotechnology should be respected. Most Biotech only have one or two core products. Super large single products have to pay for countless failures in the past, and also support the next wave of research and development that may fail countless times. Without large single products, they cannot form an innovation cycle, otherwise becoming a project production company is not as certain as film and television companies.
This means that Biotech's core products bear both the task of recovering its own R & D costs and the task of paying for all the R & D costs of the enterprise.
Both tasks are difficult to accomplish. Despite the extreme situation of Tengsheng Bo medicine 1.4 billion yuan research and development neutralization antibody only sold 51.6 million yuan, let's look at PD-1, which reflects the universal fate of innovative drugs. According to the statistics of Yixi Weibo, the r & d cost of three domestic PD-1 models in China is between 2 billion and 5 billion yuan, among which, the first approved Triprizumab has a cumulative r & d investment of 4.732 billion yuan, and the cumulative sales in five years is 3.848 billion yuan. Rongchang biological double core products Thai west, d west stop commercial in 2021,3 years total product revenue of 1.9 billion yuan, the total research and development spending 3 billion yuan, the former cannot cover the latter, among them, the 2023 product income 1.049 billion yuan, research and development spending 1.306 billion yuan, after three years of sales climbing, product income still cannot cover r & d spending.
The cost of drugs is not limited to research and development expenses, but also includes the cost of raw materials, labor, manufacturing, and promotion and distribution.
In the case of linear growth of product revenue cannot bear the weight of innovation, overseas large BD has become the only option for survival.
Anxiety is still spreading among investment institutions.
The door of Hong Kong stocks has not been closed, but the undertaking capacity is insufficient. In the bear market in 2022-2023, the annual fundraising is about HK $3.5 billion, down 90% compared with the bull market in 2020-20-2021, and the primary market investment valuation in 2021-2022 and the current secondary market valuation are inverted.
The number of listed pharmaceutical companies in 2021,2022 and 2023 is 20,13 and 2 respectively. Among them, Baili Tianheng and Zhixiang Jintai officially landed on the science and Innovation Board on January 6 and June 20,2023 respectively. Since Zhixiang Jintai raised 3.473 billion yuan in its IPO, which caused huge controversy, the science and Technology Innovation Board has closed the door to unprofitable biotechnology.
In March 2022, Zhixiang Jintai terminated the research and development of two projects, namely GR1405 targeting PD-L1 and GR1401 targeting EGFR, both due to the "fierce drug competition" of the same kind, with the cumulative investment of 166 million yuan and 53.26 million yuan respectively.
This is a small wave of China's innovative drugs, but indirectly paid by the secondary market investors. Zhixiang Jintai plans to use 842 million yuan to raise funds to supplement the working capital. The terminated PD-L1 was originally commissioned for molecular development, and the controversial DDSU model came to an end.
The early innovation of biotechnology relies heavily on capital, and when VC / PE lacks exit channels, the investment and financing will be immediately frozen. According to the data of the Science and Technology Innovation Board Daily Venture Capital, the financing scale of the domestic medical and health field in 2023 totaled 53.224 billion yuan. In contrast, the total financing scale of the medical and health sector in 2022 is 397.634 billion yuan.
The long-term innovation capacity of biotechnology will be compromised.
At the end of last year, Zero 2 ko Research Center conducted research on VC / PE institutions, among which the most concerned issues changed from fundraising to exit. From 2015 to 2017, China's VC / PE market entered a period of explosive growth, and RMB funds were established in batches. Considering the general seven-year duration of the early government guide funds, the last two years are facing a wave of fund liquidation, and the industry has entered the exit cycle.
In desperation, the hand stretched out to the overseas straw.
The biotechnology dividend released by the resonance of medical reform and overseas scientists' entrepreneurship has been released, and an accurate positioning should be given. Is medicine a consumptive industry, constantly consuming payment capacity, hospital resources, or an emerging industry that creates incremental growth. We should encourage and give priority to the allocation of resources?
Be careful of India's emergence of a transition from a generic power to innovative drugs. India's four largest CDMOs are competing with Chinese companies in the small molecule sector, with the TOP10 pharmaceutical maker Glenmark, which officially entered the innovative drug track in January.
Can't drag.




