Gene therapy is a therapeutic modality that introduces a target gene into target cells and treats or prevents disease through the regulation, repair, replacement, addition or deletion of genetic sequences. Gene therapy has developed rapidly in the past 20 years, and medical scientists hope to cure various genetic diseases through gene therapy. Today, the delivery of target genes by adeneno-associated virus (AAV) to the retina, liver and nervous systems, can improve the clinical symptoms of patients with congenital blindness, hemophilia B and spinal muscular atrophy, respectively.
1. The eye is an ideal target organ for gene therapy
Gene therapy can provide sustained benefits to human health, and there are reasons to remain optimistic and increase efforts to make gene therapy a part of standard care, especially in ophthalmology, with clear advantages and rapid development (Figure 1). The eye is an ideal target organ for gene therapy. The presence of the blood retina barrier and the blood chamber water barrier 2 sets of the blood eye barrier makes the immune system of the eye relatively independent and, as a partial immune exempt organ, with a higher feasibility of gene therapy due to the suppressed immune response.
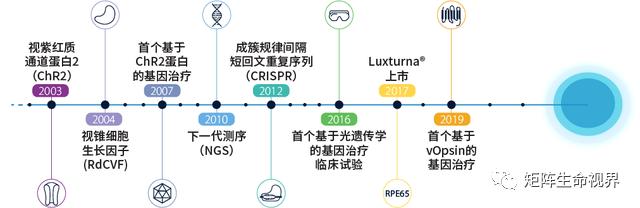
Figure 1. Review of the history from finding genetic mutations to gene therapy
Note: vOpsin is a seven-fold transmembrane protein, belonging to the G protein-coupled receptor family (GPCRs), and is usually used in the study of vertebrate optometry.[Prog Retin Eye Res.2022; 86: 100975.]
From a research perspective, the transparent structure of the anterior part of the eye allows the assessment of the retina by non-invasive examination techniques, which also makes the retina a major targeted tissue for ophthalmic gene therapy. This feature can not only diagnose and identify retinal diseases, but also assess the efficacy and safety of treatment. Depending on the target cells, the retinal administration routes can be divided into two types (Figure 2-3).
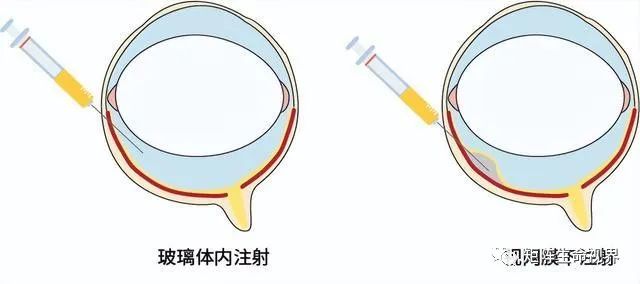
Figure 2. Route of ophthalmic gene therapy administration
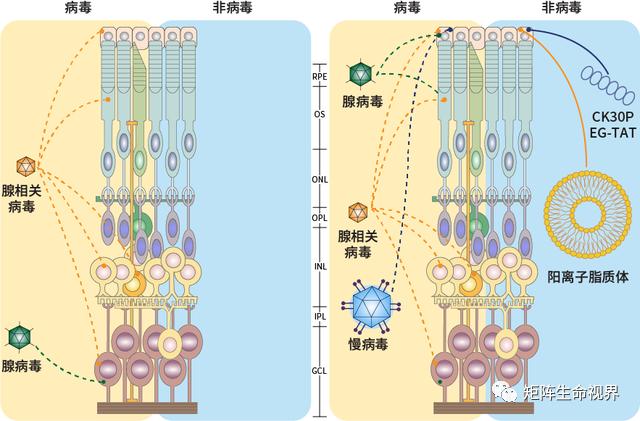
Figure 3. Delivery carrier for gene therapy in ophthalmology
Note: RPE: retinal pigment epithelium; OS: outer photosensitive layer; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner core layer; IPL: inner plexiform layer; GCL: ganglion cell layer.[Prog Retin Eye Res.2022; 86: 100975.]
When the target cells are ganglion cells and inner core layer cells, vitreous injection is preferred; for cells of the photoreceptor or retinal pigment epithelium, the advantage of the injection site is after the blood retinal barrier, but the disadvantage is more invasive, requiring retinal dissection and subretinal haemorrhage at the injection site.[Hum Gene Ther.2022; 33(17-18): 865-878.]
2. The first ophthalmic gene therapy drug is available ($42,5,000 per eye)

voretigene neparvovec
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved the first gene therapy drug in ophthalmology between 2017 and 2018, voretigene neparvovec (Luxturna®, Spark Therapeutics), For the treatment of inherited retinopathy diseases caused by mutations in the double-copy RPE 65 gene, The RPE 65 gene is responsible for encoding the enzyme that transforms the all-trans-retinoid to 11-cis-retinol, This process is part of the visual cycle within the retinal pigment epithelium, When the RPE 65 gene is mutated, Causing such diseases to progress to legal blindness, It also means that gene therapy has the potential to achieve a true "comeback".
Phase 1-2 of Voretigene neparvovec (VN) related clinical trial determined the safety of the drug, and most subjects had visual improvement of more than 15 letters; the result of the phase 3 clinical trial was the first randomized controlled trial of ophthalmic gene therapy, and the mobility test performance of 20 patients in the intervention group was better than 9 patients in the control group, and the efficacy remained stable within 1 year. And because the critical phase 3 data showed statistically significant improvements in functional vision, Luxturna® became the first approved ophthalmic gene therapy drug.[Chinese Journal of Fundus Diseases, 2021,37 (11): 896-900.]
The VN delivers the correct coding sequence of the human RPE 65 gene in the AAV vector to the retinal pigment epithelium by subretinal injection. The basic procedure is as follows: After vitrectomy, a small injection sleeve and slight pressure creates a retinal incision, through which the injection can be passed into the subretinal space to complete the delivery of the treatment carrier solution (Figure 4). VN treatment can be done by the surgeon manually or by controlling the foot pedal injection device. Patients received a single injection of 1.5 x 1011 vector genome per eye with an expected target volume of 300 μ L.
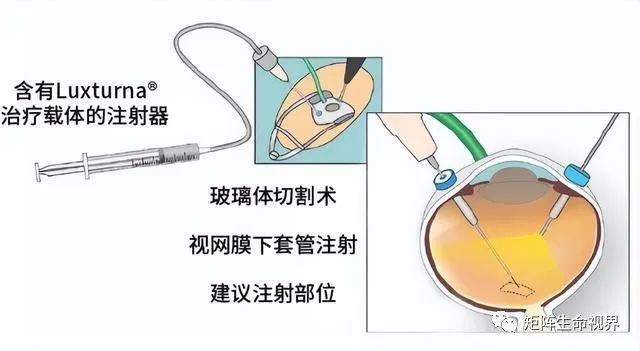
Figure 4. Luxturna® Source: ophthalmology times & LMU Eye Hospital
One or more fluid-filled air bubbles (blebs) form under the retina after injection. These bubbles were absorbed within 24 – 48 hours of subretinal administration. Drugs are mediated by the receptor-mediated uptake by the target cell retinal pigment epithelial cells. Once in the nucleus, single-stranded DNA is transcribed into double-stranded DNA, and the mRNA is subsequently translated into a functional protein in the cell body.
3. Recent case reports of related retinal atrophy
New gene therapies such as this offer hope for incurable diseases, however, as with any entirely new therapeutic product, real-world data on new gene therapy is very limited, so uncertainty about drug durability, safety, and benefit-to-risk ratio naturally exists. Recent case reports of postoperative retinal atrophy are indeed alarming. In the long run, VN may cause potentially destructive retinal outcomes, and further studies are needed to follow up on the potential long-term safety concerns of gene therapy.
In early clinical studies, there are also some risks of gene therapy. But the vast majority of treatment-related adverse events are transient and mild, including elevated intraocular pressure, cataract, ocular inflammation, retinal tears, foveal formation (dellen), retinal deposition, etc. Common complications of subretinal injection therapy include: peripheral retinal cracks, secondary macular holes, retinal haemorrhage, and retinal detachment.
A study published in 2022 reported that a total of 18 eyes had peripheral chorioretinal atrophy, and 8 of 10 (80%) patients had bilateral ocular atrophy. It started 4.7 months after surgery, and the range gradually expanded in all cases with a mean follow-up period of 11.3 months (4-18 months). Ten eyes (55.5%) had atrophy both medial and outside of the subretinal injection area (blebs), seven (38.9%) only within the injection area and one (5.5%) only in the injection area. Despite the atrophy, the visual function of most patients remained stable or even improved. Twelve of the 18 eyes had vision improvement, three were unchanged, and three had vision loss. After statistical analysis, there was no significant change in mean visual acuity before and after surgery (P=0.45).[Ophthalmol Retina.2022; 6(1): 58-64.]
Another study this year also reported that retrospectively analyzing eight patients receiving subretinal injection of VN had retinal atrophy in 13 eyes 6 – 24 months after surgery. All patients developed atrophy within the injection area, and three patients developed atrophy outside the injection area. The area of atrophy expanded over time, but the visual outcome (visual field) remained stable.[Br J Ophthalmol.2022; bjophthalmol-2021-321023.]
Gene therapy subretinal injection of VN can cause retinal atrophy that may cause loss of photoreceptors inside and outside the injected area. However, two recent reports showed that although the area of atrophy also included the central area, the functional benefits of the treatment were not affected, and the visual function remained relatively stable. The possible reasons for retinal atrophy after gene therapy should first consider the immune factors. The immune response against the vector genome or against the shell may also be related to the related impurities incorporated in drug manufacturing.
Secondly, consider the surgical factors, that is, the stress response occurred due to the retinal damage caused by the mechanical external force at the injection site. Meanwhile, patients with RPE 65 gene-related retinal diseases have dystrophic retinal changes, and more vulnerable retinas may be more vulnerable to injury.
4. Gene therapy requires standardized therapy to reduce the risk
In August 2022, the Chinese medical association of ophthalmology fundus pathology group issued " Chinese genetic retinal disease gene therapy surgery safety management and visual function evaluation index set expert consensus, for genetic retinal disease gene therapy perioperative management specification consensus opinion (figure 5), for ophthalmology and related discipline clinicians reference in clinical practice, in order to standardize treatment, reduce risk, to better protect the interests of patients.

Figure 5. Flow chart of the perioperative management of IRD gene therapy
Note: IRD: inherited retinal disease; VA: vision; OCT: optical coherence tomography; CF: Color fundus photography; AF: autofluorescence; VF: visual field; ERG: Electroretinogram; EOG: electro-oculogram; VEP: Visual-evoked potentials; FST: Full-field photosensitivity threshold test; MLMT: Multi-brightness mobility test; RP: retinitis pigmentosa; LCA: Leber congenital dark; EORP: Early hair-style RP; RS: congenital retinal split disease; BEST: BEST vitelloid macular dystrophy; STGD: adolescent macular malnutrition; PCR: Polymerase chain reaction; PBMC: peripheral blood mononuclear cells.[Chinese Journal of Fundus Diseases, 2022,38 (8): 636-642.]
sum up:
Treatment number of ophthalmic gene therapy is very limited, the real world data is insufficient, is not enough for the development of postoperative retinal atrophy and the influence of long-term visual function to a clear conclusion, but the specification of gene therapy related research and long-term follow-up for all patients is very important, the latter can make the doctor may have a more accurate understanding of the long-term impact of treatment. This has important implications for future patient selection and helps to predict the expected treatment effect of eligible patients.




