On April 3,2024, the US Food and Drug Administration (FDA) announced the approval of Basilea Pharmaceutica's own new molecular entity, Zevtera (active ingredient: Ceftobiprole Medocaril Sodium). Zevtera For the treatment of adult patients with Staphylococcus aureus bloodstream infection (bacteremia) (SAB) (including patients with right-side infectious endocarditis), adults with acute bacterial dermatitis and skin structure infection (ABSSSI), and community-acquired bacterial pneumonia (CABP) in adults and children (3 months to 18 years of age).
Zevtera For the treatment of bacteremia (SAB)
Staphylococcus aureus bloodstream infection (bacteremia) (SAB) is the presence of bacteria in the bloodstream. It may occur spontaneously via the urinary tract or venous intubation, or it may be secondary to the oral cavity, digestive tract, urinary tract or trauma care or other procedures. Bteremia may lead to migratory infections, including endocarditis, especially in the presence of heart valve lesions. Recessive bacteremia is usually asymptomatic but can lead to fever. Other symptoms that often indicate more severe infections, such as sepsis or septic shock.
A randomized, double-blind, multi-center phase III clinical study (NCT03138733) evaluated the efficacy and safety of Zevtera compared to Daptomycin (daptomycin) in the treatment of Staphylococcus aureus bacteremia (including infective endocarditis). This clinical trial was conducted by Basilea on 26 August 2018 and completed on 11 March 2022. A total of 390 patients with Staphylococcus aureus bacteremia were enrolled. In the trial, the investigators randomassigned 192 subjects to receive Zevtera and 198 subjects received Daptomycin (daptomycin) as a control. Zevtera Invenously every 6 hours for the first 8 days after initiation and intravenous every 8 hours thereafter, and Daptomycin (daptomycin) control group every 24 hours. The primary efficacy measures were overall treatment success at 70 days after randomization, including survival, symptom reduction, flow clearance of S. aureus, absence of new S. aureus bacteremia-related complications, and absence of other potentially effective antibiotics.
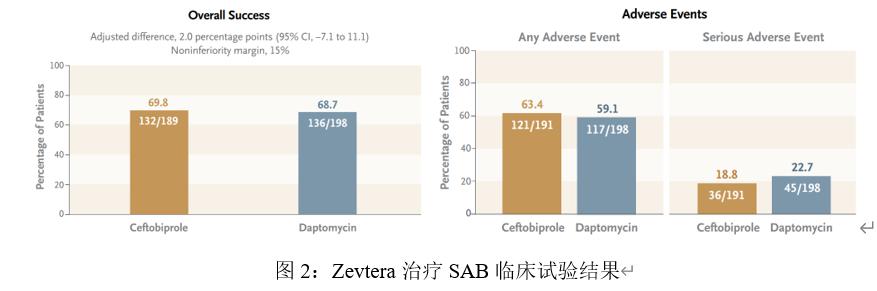
Clinical results showed that in effectiveness, 69.8% of subjects receiving Zevtera achieved overall success compared with 68.7% of subjects receiving control therapy; for safety, the rates of adverse reactions or serious adverse reactions were similar in both groups. The jects in the Zevtera group were slightly higher than in the Daptomycin (daptomycin) group. These data suggest that Zevtera is no less effective than Daptomycin (daptomycin).
Zevtera For the treatment of ABSSSI
Acute bacterial dermatitis and skin structure infection (ABSSSI) is a complex and complete bacterial infection of the skin, Currently, the main symptoms of ABSSSI include cellulitis, erysipelas, lymphangitis, Over the past 20 years, A progressive increase in the number of ABSSSI patients diagnosed worldwide, ABSSSI Common complications include secondary joint infections, bloodstream infection (BSI), and amputation, Of which BSI is considered as one of the most serious complications, Bacteria circulate the blood, Severe patients can cause multiple organ failure or cause infectious septic shock, threat to life.
A randomized, double-blind, multicenter phase III clinical study (NCT03137173) compared the efficacy and safety of Zevtera and Vancomycin (vancomycin) for ABSSSI. The clinical trial was conducted on 19 February 2018 and completed on 22 April 2019.679 ABSSSI patients were enrolled, with 335 subjects receiving Zevtera and 344 subjects receiving Vancomycin (vancomycin) in combination with tretreonam. The primary efficacy endpoint of this trial is early clinical response 48-72 hours after initiation in the intent-to-treat (ITT) population. Early clinical response requires a reduction of at least 20% of primary skin lesions, survival of at least 72 hours, and no additional antimicrobial therapy or unexpected surgery.
The results showed that the early clinical success rate in the Zevtera treatment group was 91.3%, compared with the 88.1% in the control group, with confirmed non-inferiority (adjusted difference: 3.3%; 95% CI: -1.2,7.8). The clinical success rate assessed by the investigator during the cure trial (TOC) examination was similar, showing non-inferiority in the ITT (90.1% vs 89.0%) and the clinically evaluable population (97.9% vs 95.2%). Treatment success rates and safety profiles were similar in both groups. These results suggest that Zevtera is no less effective than vancomycin / tretreonam.

Figure 3: Results of Zevtera for ABSSSI
Zevtera For the treatment of CABP
Community-acquired bacterial pneumonia (CABP) is a common and serious infection. One in five patients with CABP is severely infected and requires hospitalization with a mortality rate of 8%. Treatment with antibiotics covering possible pathogens should begin within hours after the diagnosis of CABP. Delayed antibiotic treatment or with ineffective antibiotic treatment can increase patient morbidity and mortality.
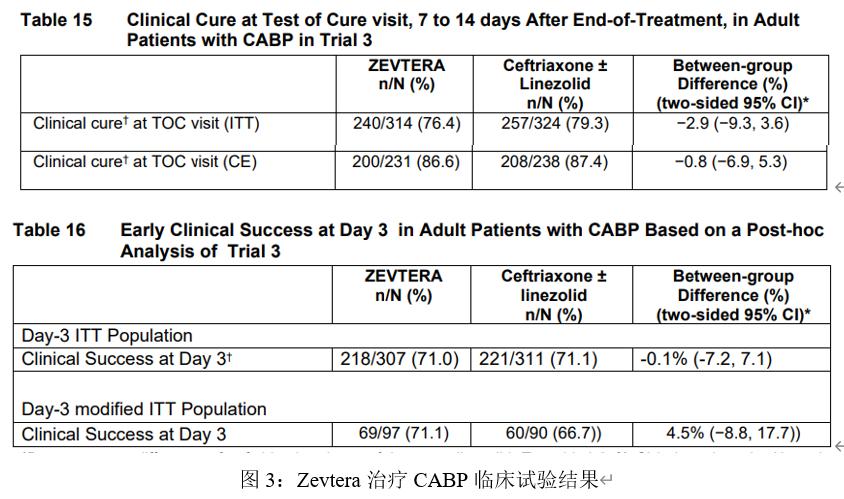
A randomized, double-blind, multi-center phase III clinical study (NCT00326287) evaluating the treatment of Zevtera for community-acquired bacterial pneumonia (CABP) trial began in June 2006 and ended in July 2007 with a total of 638 subjects enrolled. Of these, 314 subjects received Zevtera, and the remaining 324 subjects received Ceftriaxone (ceftriaxone). The primary measure of efficacy is the clinical cure rate test performed 7-14 days after the end of treatment.
The results showed that 76.4% received Zevtera, and 79.3% received control treatment. Another analysis considered an earlier day 3 clinical cure time point, where 71% of patients receiving Zevtera achieved clinical cure compared with 71.1% of patients receiving control therapy.
These results suggest that intravenous Zevtera is no less effective in combination with Ceftriaxone (ceztriaxone) in patients with pneumonia in the community and without linezolid (linezolid).
Zevtera Clinical course
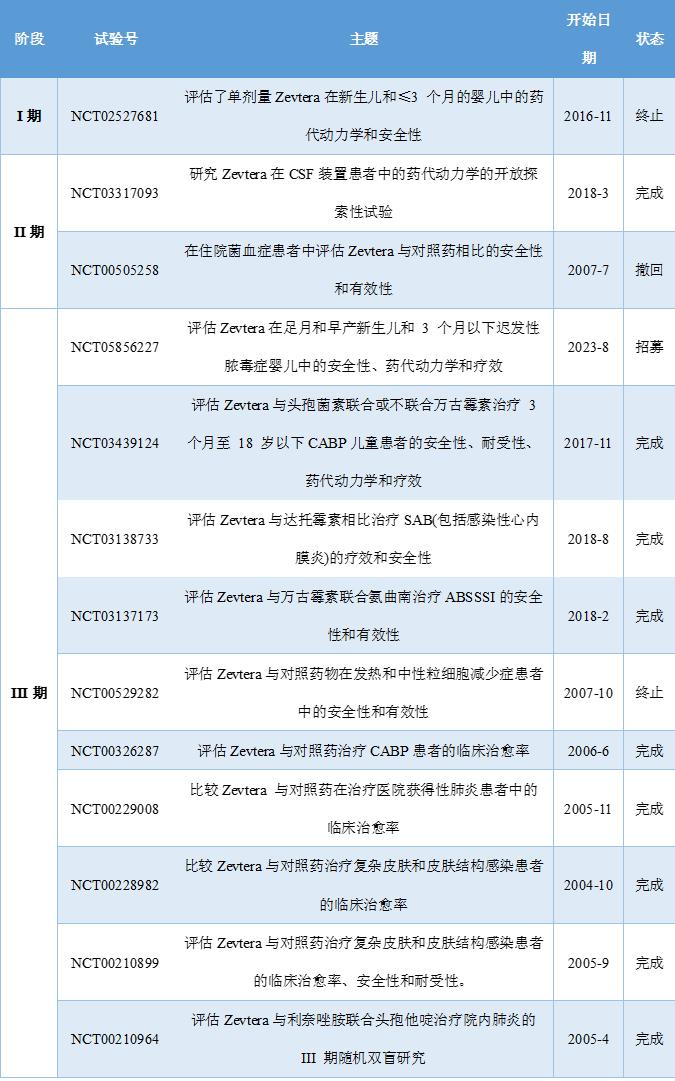
This FDA approval is based on the positive results that Zevtera showed in 3 pivotal phase III clinical studies, Are the NCT03138733, NCT03137173, and NCT00326287, respectively, These 3 clinical studies have evaluated the safety and efficacy of Zevtera for the treatment of patients with SAB, ABSSSI, and CABP, respectively, In addition, dozens of clinical studies in Zevtera worldwide, Fully evaluated its pharmacodynamics, pharmacokinetics, safety, efficacy, tolerability, Some of the important clinical studies of Zevtera are summarized below.
Sum up
Zevtera Is a new generation of intravenous cephalosporin antibiotic that quickly kills a variety of Gram-positive bacteria (e. g. Staphylococcus aureus, including methicillin-resistant strains) and Gram-negative bacteria."The FDA is committed to promoting the supply of new antibiotics proven safe and effective, and Zevtera will provide additional treatment options for some serious bacterial infections," said Peter Kim, director of the Anti-infective division at the FDA Center for Drug Evaluation and Research."
reference material:
[1]FDA official website: https: / / www.fda.gov/
[2] Basilea Website: https: / / www.basilea.com/
[3] Holland TL, Cosgrove SE, Doernberg SB, et al.Ceftobiprole for Treatment of Complicated Staphylococcus aureus Bacteremia.N Engl J Med.2023;389(15):1390-1401.
[4]Overcash JS, Kim C, Keech R, et al.Ceftobiprole Compared With Vancomycin Plus Aztreonam in the Treatment of Acute Bacterial Skin and Skin Structure Infections: Results of a Phase 3, Randomized, Double-blind Trial (TARGET).Clin Infect Dis.2021;73(7):e1507-e1517.




