At the AACR 2024 annual meeting, the latest research on PROTAC degraders has included several noteworthy developments. These studies cover not only the development and design of PROTAC degraders, but also their applications in targeted cancer therapy.
From ADC to DAC: PROxAb Introduction
At this year's AACR annual meeting, PROTAC Pioneer Arvinas sponsored a feature called "From Chemistry to Clinical" focusing on PROTAC and ADC, as well as the technological breakthroughs brought about by the combination of these two areas.
The report of Marcel Rieker, a scientist from Merck, Germany, has attracted much attention and discussion. His report revolves around an exciting concept: combining two frontier drug discovery fields, ——PROTAC and ADC, to explore a new strategy to deliver degraders to diseased cells.
Marcel Rieker The PROTAC-ADCs (antibody drug conjugate) and self-assembled PROxAb shuttles introduced at the AACR 2024 annual meeting represent an important innovation of PROTAC technology in the field of drug delivery system design. Through this study, it shows how to improve the selectivity and efficacy of drugs for specific targets, which is of great significance for cancer treatment.
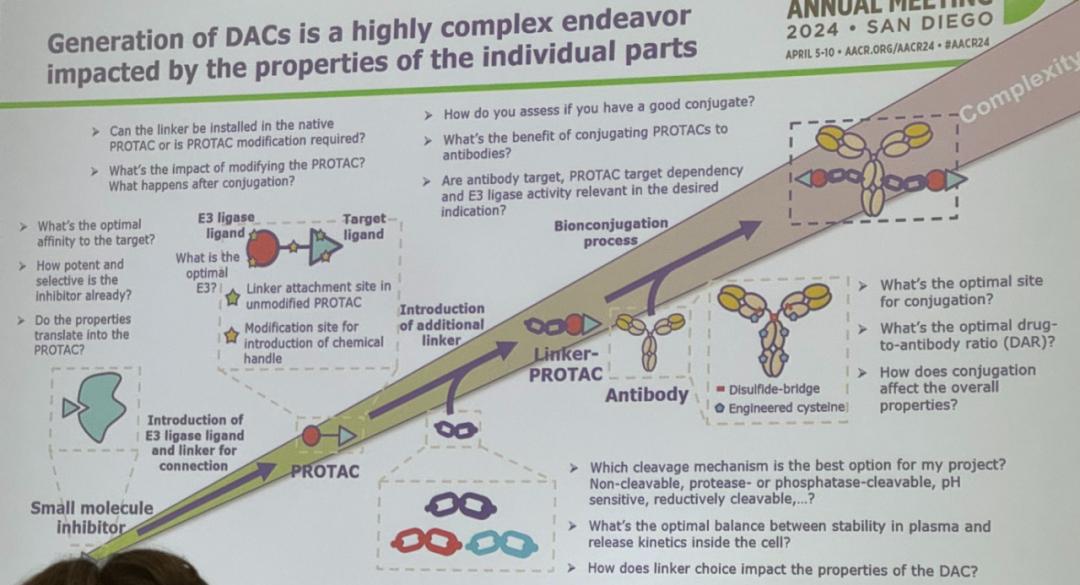
Figure 1 This slide shows the process of generating Degrader Antibody Conjugates (DACs, degrader antibody conjugate).
Photo credit: AACR meeting screenshot
From the above, we can understand the generation process of degrader antibody conjugates (DACs), which reveals the high complexity and the key factors affecting their development.
The design of DACs starts with small molecule inhibitors, and their effectiveness, selectivity and applicability of PROTAC (directed protein degradator) technology need to be considered in the early stage of development. Subsequently, the investigators need to introduce the E3 ligase ligand and linker into the system to link the inhibitor to the PROTAC. In the construction of DACs, determining the optimal attachment point of the connector on the PROTAC and whether modifications to the PROTAC are required is an essential step. Specific chemical modifications to the PROTAC may also be necessary to further introduce the chemical handles.
In the antibody part, determining the optimal coupling site and the drug-to-antibody ratio (DAR) is essential to ensure the overall nature of the conjugate. The bioconjugation process involves binding the antibody to the connector-PROTAC, which is a critical step in forming the final DAC product. The complexity of the process increases with the conversion from the individual components to the complete conjugate.
Several important issues are also addressed in the figure, how to assess the quality of the conjugated product, the benefit of conjugating PROTAC to antibodies, and the correlation between the activity of the E3 ligase and the indication pursued. Furthermore, researchers must decide on the lysis mechanism and find the optimal balance between plasma stability and intracellular release dynamics. Ultimately, the influence of the choice of the connector on the DAC properties is also a key factor that cannot be ignored. Different types of linkers such as disulfide bridges and modified cysteines are also mentioned in the slides, choices that would directly affect the cleavage mechanism of DAC and its potency in therapeutic applications.
In ADC, disulfide bridges and modified cysteines are two key components that play important roles in drug stability and release.
Disulfide bridges (Disulfide Bridges): These are the sulfur-sulfur bonds within the antibody molecule, usually found between the heavy and light chains of the antibody, and in the hinge region of the heavy chain. In the design of the ADC, these disulfide bonds can be used as connecting points to couple the drug payload to the antibody. This coupling manner can expose the free cysteine residues by reducing the disulfide bond, thus providing the available sites for the coupling of the linker payload with the antibodies.
Modiformed cysteine (Engineered Cysteines): Through genetic engineering, new cysteine residues can be introduced at specific locations of antibodies. These specially added cysteine residues provide additional conjugation sites, allowing the drug to bind to the antibody in a more homogeneous manner. This approach helps to reduce the heterogeneity of ADC and improve drug stability and therapeutic efficacy.
In the development of ADC, selecting the appropriate junction site is critical because it directly affects the drug release and the antibody function. Disulfide bridges and modified cysteines provide a means to effectively couple a drug payload to an antibody without compromising the antibody structure and function. The development and application of these technologies make ADC a promising strategy for cancer treatment.
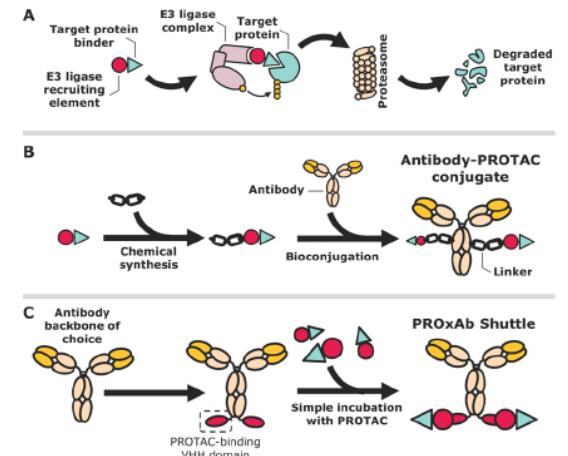
Figure 2 Comparison of the non-covalent PROxAb Shuttle technique with the covalent antibody-PROTAC conjugate. A) Mode of action of the proteolytic-targeting chimera (PROTAC). B) Covalent ligation of PROTAC with the antibody depends on finding a suitable exit vector on the PROTAC for ligation. Subsequently, the corresponding linker-PROTAC molecule is required to conjugate to the antibody backbone. C) PROxAb Shuttles relies on single-domain antibodies from camel ids that bind to the VHL ligand-binding subunit of PROTAC. By using this antibody domain with an existing antibody scaffold, a dual specific fusion protein is available.
Photo source: Reference paper 1
Another highlight of their preprint paper is that it does not choose traditional antibodies, but rather use nanoantibodies (nanobody) in camels and alpacas. Compared to conventional antibodies, nanoantibodies possess longer active binding regions of up to 16 to 18 amino acids. The real charm of nanoantibodies lies in their excellent temperature stability and strong tolerance to organic solvents, which gives them an unparalleled advantage in drug development and delivery.
More importantly, when these nanoantibodies are used as part of the PROxAb shuttle body, they can not only precisely deliver small molecule drugs such as PROTACs to diseased cells, but also keep their structure and function intact in the process. This means that, even in complex organismal environments, nanoantibodies can ensure the high efficiency and precision of drug delivery, bringing new hope for disease treatment.
STAT3 PROTAC A New chapter of the degrader
STAT 3, whose full name is signal transduction and activator of transcription 3, belongs to a key member of the STAT family. It activates the expression of downstream genes, in turn, and regulates key processes such as cancer cell survival, proliferation, neovascularization, invasion, metastasis, drug resistance and immune escape. The aberrant activation of STAT 3 in multiple human diseases, especially cancer, makes it a potential target for the treatment of these diseases. However, the drug development of small molecules for STAT 3 has long been hampered by problems such as insufficient specificity.
On November 11,2019, Professor Wang Shaomeng and his team from the University of Michigan published a pioneering study in the journal Cancer Cell. This study used PROTAC to design a novel small molecule able to specifically degrade the STAT 3 protein in cancer cells in vitro and in vivo. This study not only opens up new drug strategies for the treatment of multiple cancers, but also provides new ideas for designing degraders using PROTAC technology for other difficult-to-drug targets. This marks the opening of the door to challenging long-term difficult targets in the field of cancer therapy.
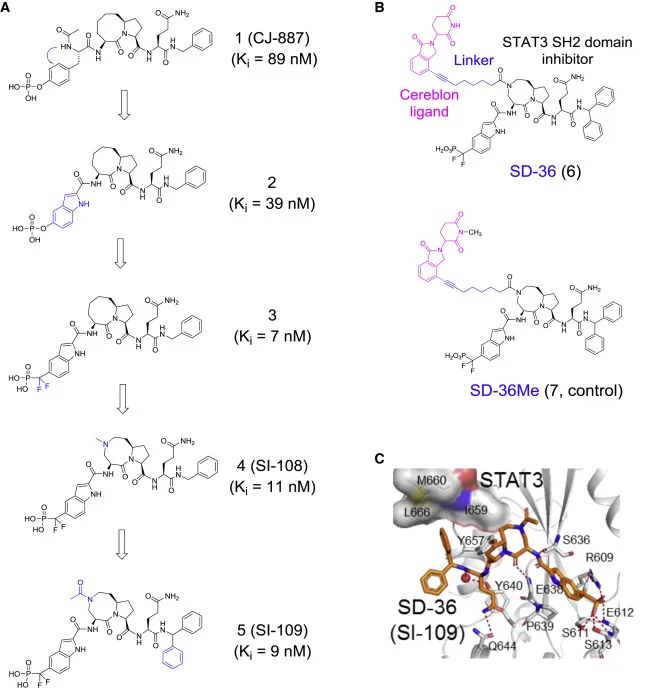
Figure 3 Structure-guided design of STAT3 SH2 domain inhibitors and PROTAC degraders (A) Design of STAT3 SH2 domain inhibitors. Ki values are the average of three independent experiments.(B) Chemical structure of the PROTAC STAT3 degradator SD-36 and its inactive control SD-36Me.(C) Co-crystal structure of SD-36 (orange) and STAT 3 (gray). The binding pattern of SI-109 to STAT3 is almost identical to that of SD-36.
Photo source: Reference paper 2
This year AACR conference they released a new generation of STAT 3 degrader UM-STAT 3-1218.
New generation compounds showed over 50-fold increased potency in cells to induce STAT 3 degradation and demonstrated more than 500-fold degradation selectivity for other STAT members.
Proteome analysis revealed that the lead compound UM-STAT 3-1218 only reduced the level of STAT 3 protein in cells and had no effect on more than 6700 other proteins. UM-STAT 3-12,18 achieved a low nanomolar grade IC50. A single intravenous dose of 3 mg / kg reduced STAT 3 protein levels over 4 days in xenografted tumors and primary tissues without reducing the levels of any other STAT members. Impressively, a single dose of 3 mg / kg UM-STAT 3-1218 achieved complete and durable tumor regression in multiple xenograft tumor models without signs of toxicity.
Conclusions and prospects
In this era, we use small molecules to open the unknown door.
Technological innovation, like the north wind at night, blows through the sails of drug discovery.
The degradation changed from whispering in class to singing in meetings.
Chemistry and protein, interwoven into an endless dream.
Chemical proteomics, like the lights of the Pathfinder, on the unknown night ship,
The first drugs, which are the seeds of hope, sprout quietly in the clinical land.
Drug candidate molecules, whose complexity acts like corals in the deep sea,
Every choice is a challenge to the abyss.
We seek not just a goal, but the light of many lives.
And this industry, like a long voyage,
From the storm of competition to the harbor of cooperation.
The battlefield of cancer is no longer an island of contention,
But hand in hand, together facing the wind and waves of the continent.
This is a new journey that we are looking for,
Not just a cure, but an understanding, is symbiosis, is harmony.
On this road, every step remembers the past,
Towards a brighter future.
reference material:
1, PROxAb Shuttle, the technical paper preprint version
DOI:10.1101/2023.09.29.558399
2, the previous generation of stat 3 degradator SD-36 paper
doi.org/10.1016/j.ccell.2019.10.002
3, AACR 2024 summary download
AACR2024_Regular_Abstracts_04-01-24.pdf
4. Details of ADC bioconjugation technology and research progress-Zhihu.
https://zhuanlan.zhihu.com / p / 383720783. Ref
5. de novo learning of antibody drug conjugation (ADC)
https://zhuanlan.zhihu.com/p/163027244.




