In recent years, the higher approval rate and shorter research and development cycle have driven the research and development enthusiasm of peptide drugs. Due to the widely suitable indication, high safety and remarkable efficacy, peptide drugs have been widely used in the prevention, diagnosis and treatment of tumor and immune diseases, and have a broad development prospect. At present, there are about 180 peptide drugs approved worldwide, mainly in the anti-tumor field (30%), followed by digestive tract (14%), diabetes (13%) and rare diseases (11%). With the rapid development of the global polypeptide drug market, the global market size will reach us $72.9 billion in 2022 and is expected to reach US $79.5 billion in 2023.
Table 1. Main varieties of polypeptide drugs for each indication

Source: Public information, Kelayin
1. Development history of polypeptide drugs
Polypeptide drugs have a long history of development. Since the first polypeptide substance was discovered in the human body in 1902, polypeptide research has been developed for more than a hundred years.1922 polypeptide (insulin) used for the first time in the human body, opened a new chapter of peptide drugs, 1963 polypeptide solid phase synthesis technology (SPPS) invention for peptide drugs brought a landmark revolution, its synthesis, quickly, become the preferred method of peptide synthesis, gave rise to the rapid development of polypeptide drugs. After the 1970s, synthetic polypeptides began to be used in clinical practice. With the iteration of polypeptide structural modification and chemical modification methods, including the modification of peptide chain skeleton, and the introduction of fatty acid, polyethylene glycol, protein fusion and other modification optimization, the problems such as poor selectivity, easy degradation and short half-life have been solved. In addition, the development of new targets, the expansion of new indications and dosage form optimization bring new increment to the market space of polypeptide drugs.
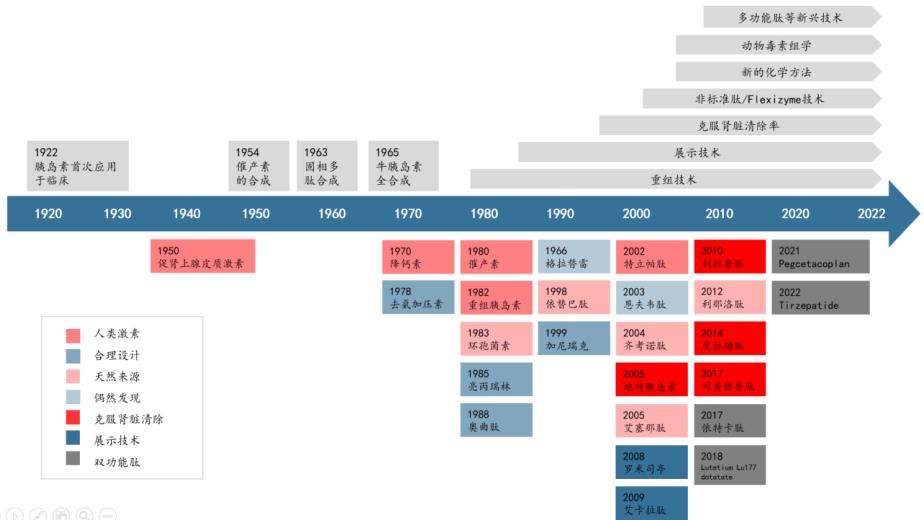
Figure 1. Development history of polypeptide drugs
Source: Nat Rev Drug Discov, CITIC Jiantou Securities
2. polypeptide drug synthesis process
Polypeptide synthesis methods can be divided into chemical synthesis methods and biosynthesis methods, and chemical synthesis methods can be divided into solid phase synthesis and liquid phase synthesis. The main difference between the two methods is whether the solid phase carrier resin is used.
Based on the advantages of high process development efficiency, convenient operation and high yield, the solid-phase synthesis method has gradually become the mainstream method of polypeptide synthesis, which is suitable for the synthesis of 10-50 amino acids. Solid-phase polysynthesis is finally obtained through the solid-phase carrier link, multi-step condensation reaction, modification, separation and purification. In the process of solid phase synthesis, the selection of solid phase carrier, the mass stability of peptide synthesis reagent, and the selection of separation and purification method all have a great impact on the yield and purity of peptide drugs.
Liquid phase synthesis is suitable for short chain peptide synthesis: liquid phase synthesis developed early, which is divided into gradual synthesis and fragment condensation: gradual synthesis is usually gradually adding connecting amino acids from the C end of the polypeptide chain until the whole polypeptide chain; fragment condensation generally synthesize each required fragment, and then condenses each fragment to synthesize the target polypeptide. The liquid phase method has been gradually replaced by the solid phase synthesis method due to the large pollution and complex purification. Due to its low unit cost, it is usually widely used in polypeptide synthesis below 10 amino acids.
Biosynthesis includes natural extraction method, enzyme catalysis method, fermentation method, and gene recombination method.
Fermentation method is to use microbial metabolism to obtain polypeptides, the advantage is low cost, but it is difficult to separate. Fermentation method can directly produce few peptide drugs, on the one hand, combined with chemical synthesis method, Nordsi megallutide is prepared by fermentation method + solid phase method; on the one hand, fermentation method is also the basis of gene recombination method, with broad application prospect.
Genetic engineering method is the future research direction: producing polypeptide drugs based on synthetic biology technology, which is suitable for the preparation of long-chain peptides and complex peptides. The advantages of genetic engineering method are expression orientation, safety and environmental protection, while the disadvantages lie in the difficulty of research and development, long cycle and low yield.
Table 2. Comparison of polypeptide synthesis methods
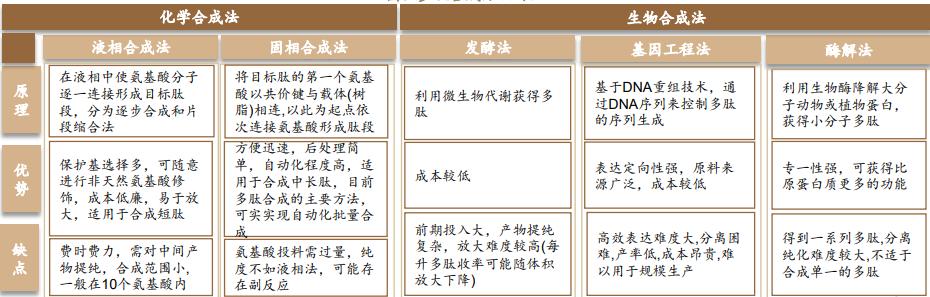
Source: Peptide Research Society, Deppon Research Institute
3. Polypeptide drug industry chain
The upstream of the peptide drug industry chain are raw material suppliers, including peptide synthesis reagent, amino acid, solid phase carrier resin, filler, solid phase synthesizer, etc.; in the middle of the industry chain are peptide drug development and production enterprises; and the downstream of the industry chain is the sales terminal, mainly for drug wholesalers to medical institutions and finally sold to patients.
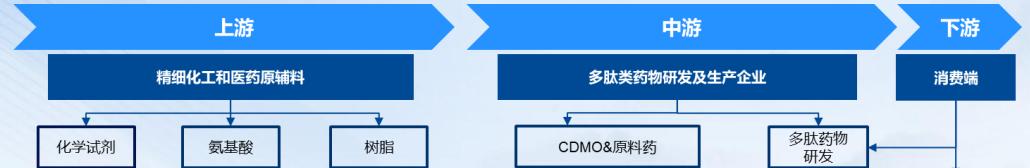
Figure 2. Polypeptide drug industry chain
Source: Open information collation
The amino acids of polypeptide drugs are linked by amide bonds, one of the most important functional groups in polypeptide drugs. Amide bond formation most often uses various condensation reagents, especially in liquid phase synthesis and solid phase synthesis. Due to the low cost of carbon diimide condensation reagent, it is the first choice for large-scale production in many enterprises. However, with the pursuit of research and development efficiency, and the wide use of solid-phase syntheses, ionic condensation reagents will usher in rapid development. At present, the condensation reagent has been developed to the fourth generation: DCC, DIC and EDC represented by carbon diimide condensation reagent; the second generation: carbon diimine condensation efficiency is not high, adding HOBt (ionic condensation agent) as an additive to improve the reaction rate; the third generation: the development of HOBt; the fourth generation: because HOBt and HOAt have flammable and explosive characteristics, transportation is extremely dangerous, the development of new Oxyma ion condensation reagent easy, safe by-product toxicity and other advantages. It is estimated that the global condensation agent market size is close to 10 billion yuan in 2027.、
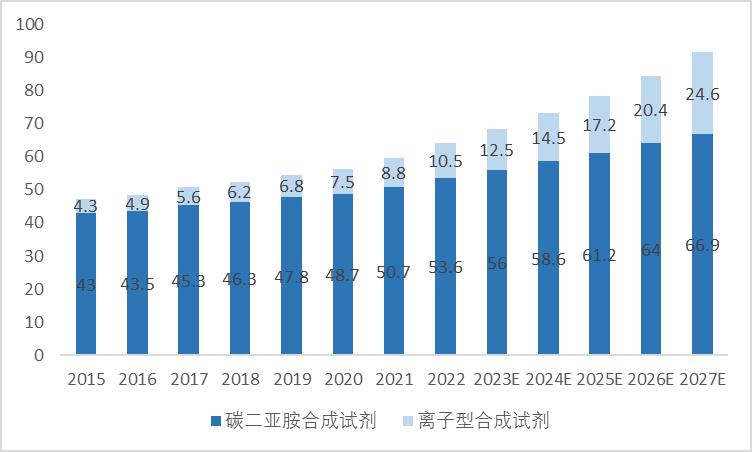
Figure 3. Forecast of global polypeptide condensation reagent market size in recent years (unit: 100 million)
Source: Peptide Research Society, Guotai Junan Securities
Amino acids are important upstream raw materials for polypeptide drugs, Its output affects the development of China's polypeptide drug industry, In 2022, China's amino acid production reached 6,282,600 tons, Year-on-year growth of 7.1%, China continues to increase its amino acid production, Providing ample raw materials for the polypeptide pharmaceutical industry, The quality of the raw material itself will affect the quality of the polypeptide drug, The fluctuations in raw material prices also directly affect the production costs of the industry, coming, With the further development of China's polypeptide drug industry chain, The number of raw material suppliers of polypeptide drugs will also gradually increase, The selection range of raw materials will be further expanded, It contributes to the long-term and stable development of the polypeptide drug industry.
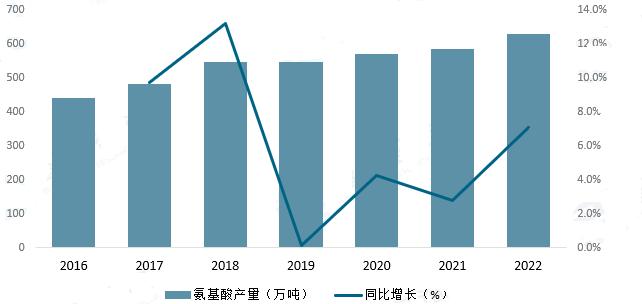
Figure 3. Statistics of amino acid production in China in recent years
Source: Zhiyan Consulting
The resin used for solid-phase polypeptide synthesis is composed of a polymer carrier and a permanently linked bifunctional group junction fragment. There are three main types of polymer resin used in solid phase synthesis: polystyrene-phenyldiethylene cross-linked resin, polyacrylamide and polyethylene-ethylene glycol resin and derivatives.
The continued expansion of the peptide drug market drives the demand for API and CDMO. According to the Polypeptide website, the global peptide API market will reach $1.8 billion in 2020, of which 65% are outsourcing services (CAGR is expected to reach 10% in 2020-2025). With the development of innovative peptide drugs, the outsourcing demand is expected to continue to expand. According to Frost & Sullivan, in 2030, the global peptide CDMO market size will reach 11.8 billion US dollars in 2030, the domestic peptide CDMO market size will reach 18.5 billion yuan, and the CAGR growth rate is higher than that of the whole world.
With the continuous deepening of scientific research and clinical practice and the increasing maturity of production technology, in the future, the application field of peptide drugs will continue to expand, and more kinds of peptide drugs will be launched, among which the proportion of innovative drugs will greatly increase, and it is estimated that the market development potential of its industrial chain is huge.
reference material:
1. Cathay Securities-gold mining 100 billion weight loss market, GLP-1 industry chain set sail by the wind-202306192.
2. Deppon Securities-pharmaceutical and biological industry peptide industry chain: weight loss indications expansion industry, investment opportunities-202306223.3. Citic Securities-Polytide synthesis reagent: Meet accelerated development opportunities-202307084.
4.https://baijiahao.baidu.com/s?id=1769119937856405280&wfr=spider&for=pc5.
5.https://www.chyxx.com/industry/1150215.html




