The first case in nearly 40 years! The FDA approved the Aprocitentan
On March 20,2024, the US Food and Drug Administration (FDA) approved the antihypertensive drug aprocitentan (Tryvio) jointly developed by Swiss company Idorsia Pharmaceutical and Johnson & Johnson. The FDA approved this drug in combination with other antihypertensive drugs for the treatment of refractory hypertension. The approval of this drug has attracted wide attention in the industry for two main reasons: (1) Apitentant reduces blood pressure by inhibiting the combination of endothelin (ET) -1 and the ETA and ETB, which is the first new antihypertensive mechanism approved by FDA in nearly 40 years; (2) it can effectively treat refractory hypertension with poor blood pressure control.
Market potential is huge! An aging population has driven soaring demand for hypertension
Hypertension is one of the most common chronic noncommunicable diseases, and it is a risk factor for stroke, coronary heart disease, heart failure, chronic kidney disease and other diseases. Epidemiological investigation show that as of 2012, the incidence of hypertension among adults aged 18 in China increased to 25.2%. With the increase of the elderly population, the incidence of hypertension increases year by year, but the control rate of hypertension is still very low. The occurrence and development of hypertension is associated with various factors such as sympathetic nervous system hyperactivity, renin-angiotensin-aldosterone system (RAAS) activation, renal excretion dysfunction, vascular endothelial damage, insulin resistance and familial inheritance. At present, the treatment of hypertension is still mainly based on drug therapy, and the marketed antihypertensive drugs mainly include diuretics, calcium ion channel blockers (CCB), β receptor blockers, angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB).
According to the data statistics of Pharmaceutical rongyun, the domestic hypertension market continued to expand from 2015 to 2019, reaching 69.092 billion yuan in 2019 (not traditional Chinese medicine). After 2020, affected by the epidemic, collective procurement and other factors, the overall market shrank to a certain extent, but the sales volume basically increased steadily.
From the perspective of market segments, calcium channel blockers and angiotensin antagonists are a high market share, with a market share of 40.13% and 31.03% respectively in 2022. The market share of β receptor blockers has gradually increased in recent years. In the future, with the influence of the aging population and the further change of living habits, the number of patients with chronic diseases represented by hypertension will continue to increase, and the demand for antihypertensive drugs will continue to increase steadily.
Domestic sales of antihypertensive drugs from 2015 to 2022
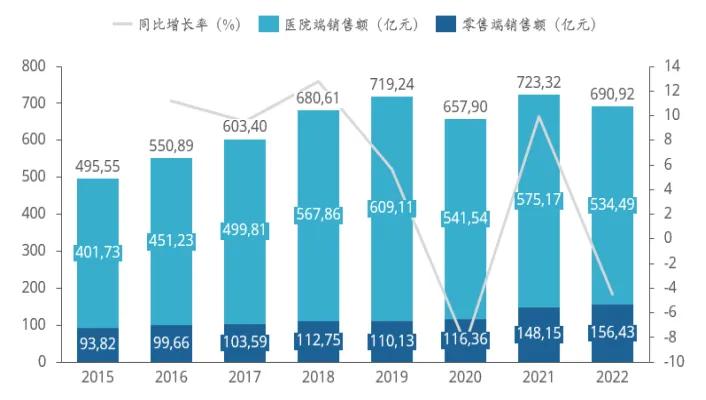
Data source: Pharmong Cloud Whole Industry Chain Database & Pharmong Consulting collation
Market proportion of main categories of antihypertensive drugs from 2015 to 2022
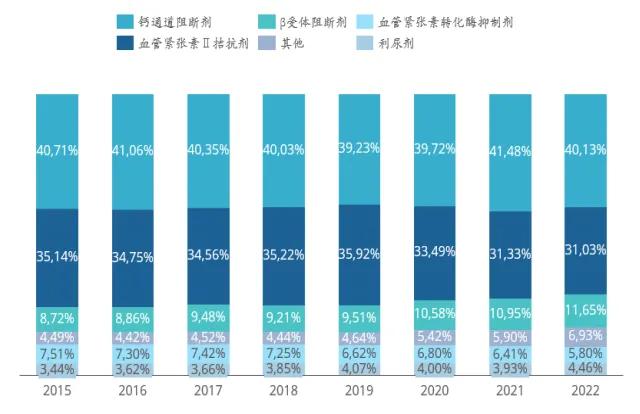
Data source: Pharmong Cloud Whole Industry Chain Database & Pharmong Consulting collation
The emergence of new mechanism of antihypertensive drugs ushered in the dawn of the treatment of hypertension
At present, the treatment of hypertension is still mainly based on drug treatment, and sufficient dose, long-acting preparation, individualized and combined application of antihypertensive drugs are the basic principles in the treatment of hypertension. However, the rate of blood pressure in hypertensive patients in China is not high, which is related to the poor efficacy of single drug treatment, the tolerability of drugs and the poor patient compliance. Therefore, the development of new and new antihypertensive drugs is of great clinical significance to improve the rate of blood pressure. At present, the development of antihypertensive drugs focus on the improvement of traditional antihypertensive drugs (such as new double channel blocking calcium channel blocker, direct renin inhibitor, dual endothelin receptor antagonist, endothelin type A receptor / angiotensin receptor subtype 1 antagonist) and explore new targets of antihypertensive drugs (such as new aminopeptidase A inhibitor, sodium and potassium channel conversion enzyme inhibitor, natriuretic peptide receptor agonist, dopamine β -hydroxylase inhibitor, membrane metal endopeptidase inhibitor, soluble guanylate cyclase stimulator), etc.
The mainstream research mechanism of action and representative drugs in the field of hypertension

Data source: Pharmong Cloud Whole Industry Chain Database & Pharmong Consulting collation
According to medicine melt cloud data statistics, the global total of 1176 hypertension related drugs (according to hypertension indications, not including marketing stage), the research and development of active state drugs a total of 310, accounted for 26.36%, IND and above drugs 112, accounted for 9.52%, it is worth mentioning that in the research termination, research suspended, no subsequent progress reports, from the inactive state of drugs, a total of 866, as high as 73.64%. However, in recent years, with the deepening of basic research and treatment of hypertension, great progress has been made in the target of new antihypertensive drugs at home and abroad, and new drugs for the treatment of hypertension have been approved, bringing new dawn to the development of this field. Due to the limited space, this paper summarizes and summarizes several progress of drug development with new mechanisms of action for reference.
01, an ETA / ETB dual endothelin receptor antagonist
Endothelin (ET) is a vasoconstrictor peptide composed of 21 amino acids. There are three types, namely ET-1, ET-2, and ET-3, which act on the vascular smooth muscle receptors ETA and ETB, which have very obvious mutual antagonistic effects. ET-1 acting on ETA can both induce strong vasoconstriction in humans and mammals, and can also activate RAAS to stimulate catecholamine release. ETA activation induces vasoconstriction, whereas ETA B activation is able to stimulate the release of endothelial diastolic factors with a vasodilatory effect that counteracts the vasoconstrictor effect of ETA.
Current studies on ET receptor antagonists (ERAs) show that ERAs can be divided into two types, namely, selective ERAs and non-selective ERAs. The current endothelin receptor antagonists in clinical use include bosentan (non-selective ERA), alicentan (selective ERA), and macitentan (selective ERA), which are mainly used for the treatment of pulmonary hypertension rather than hypertension. Blockade of ETA alone may lead to excessive stimulation of ETB receptors, causing non-selective vasodilatation, increased vascular permeability, aggravation of peripheral edema, and increased plasma vasopressin and aldosterone levels. ETA blocking was shown to significantly lower SBP and DBP as compared with placebo, but also at a significantly increased risk of edema and fluid retention. Therefore, researchers turn to drugs that selectively target the ETB receptor rather than the ETA receptor.
The Aprocitentan described above is a new oral ETA / ETB dual receptor antagonist developed by Quantum Genomics SA, with 16 times higher affinity for ETB receptor and a half-life of up to 44 h. It neither interferes with bile salt secretion nor hepatotoxicity, providing a new idea for antihypertensive treatment in patients with refractory hypertension.
02, direct renin inhibitors
RAAS plays an important role in the development and development of hypertension. Renin secreted by renal balloon cells changes the angiotensin ogen synthesized by the liver to angiotensin I (AngI), which is converted to angiotensin (Ang) under the activation of angiotensin converting enzyme (ACE). Ang mainly acts on angiotensin receptor subtype 1 (AT1), which has the effect of promoting vasoconstriction, water and sodium retention, enhancing the activity of sympathetic system and leading to rising blood pressure. Direct renin inhibitors are able to directly inhibit renin production, reduce the Ang concentration in vivo, and antagonize the pressor effect of Ang.
Alisilen is the only marketed direct renin inhibitor, which is safe and effective in treating hypertension alone or in combination with other drugs. However, aligilen has relatively low oral bioavailability and long synthetic pathway, and patients may have serious adverse effects in clinical practice. Academia Sinica, Shanghai Pharmaceutical Holding Co., LTD. (China), and Mitsubishi Tamabe Pharmaceutical Corporation (Japan) jointly developed a new generation of direct renin inhibitor SPH3127, which reached the primary study endpoint set in the Phase III (SPH3127-301) in May 2023. A total of 828 patients were enrolled in the study under a protocol of 100mg / SPH3127 tablet, taken orally once daily for 12 weeks. The results of the trial showed that SPH3127 could reduce msDBP and be safe and effective, suggesting that SPH312 tablets can provide a new treatment route and a richer treatment combination for patients with essential hypertension. In addition, the research and development team is also actively expanding the new indications for SPH3127, of which the diabetic nephropathy indication is in clinical trials in China, and the ulcerative colitis indication is also in clinical trials in China and the United States.
03, sodium and potassium ion channel conversion enzyme inhibitors
In the basolateral side of the renal tubules, the Na + -K + pump is the driver of sodium reabsorption in the kidney. Changing the Na + -K + pump function can change the systemic blood volume, and then lead to hypertension. Therefore, the renal tubular Na + -K + pump is a potential novel target for the treatment of hypertension. We have shown that two factors affecting renal Na + -K + pump function, the salt-regulating hormone endogenous waabin and the mutant cytoskeletal protein α -adducin. The above two factors promote water and sodium retention by abnormally activating the Na + -K + ion pump, and cause hypertension. Thus, drugs developed for both targets are resistant to the effects of waabain and mutant α adducin on Na + -K + pump function.
Rostafuroxin Is a safe and effective digitoxin derivative, currently in the clinical phase, Lee's Pharmaceutical has been licensed to develop and commercialize Rostafuroxin in Greater China in January this year. Rostafuroxin Acting on the Src-SH2 domain can selectively inhibit Src activation and inhibit Na + reabsorption by renal tubules by inhibiting related signaling pathways, thus reducing blood pressure. The results of the PEARL-HT clinical trial showed that Rostafuroxin had no significant effect on SBP in Chinese subjects compared to placebo; therefore Rostafuroxin may be used to guide drug treatment for primary hypertension in Caucasian patients, but further studies is still needed for Chinese essential hypertension patients.
04, Novel aminopeptidase A (APA) inhibitor
APA and aminopeptidase N (APN) are two membrane-bound zinc metalloproteinases that are involved in the metabolism of brain Ang and Ang, respectively. APA cleaves N-terminal aspartate from Ang to form Ang, while APN cleaves N-terminal arginine from Ang to form angiotensin (Ang). Ang and Ang have similar affinity to brain Ang receptors, both causing elevated blood pressure through activation of sympathetic activity, inhibition of the baroreflex at the level of the solitary tract nucleus, and increasing the concentration of arginine vasopressin in the blood. In animal experiments, the ventricle was injected with selective APA, and the inhibitor EC33 was able to cause a drop in blood pressure, suggesting that EC33 could block the baroreflex of Ang, and that Ang had a stronger pressor effect than Ang in the brain.
The novel aminopeptidase A inhibitor Firibastat is a first antihypertensive drug developed by Quantum Genomics, USA. By delivering EC33 products in the brain and specifically inhibiting aminopeptidase A, thus reducing the production of Ang and reducing blood pressure. It has been shown that it can lower blood pressure by reducing systemic vasopressin levels, reducing sympathetic tone and stimulating the baroreflex. A multicentre, open-label phase 2b study enrolling 256 patients with essential hypertension demonstrated that Firibastat was effective. However, in a multicenter (11 countries, 75 centers), randomized, double-blind, placebo-controlled, phase study (FRESH trial), failed to demonstrate effective Firibastat reduced AOBP results in patients with refractory hypertension; all subgroups were consistent with the secondary endpoints; Firibastat patients were well tolerated, the most common skin reaction with an incidence of 5.1%.
epilogue
With the aggravation of aging, the incidence of hypertension in China will be more and more high. The country has improved the accessibility of hypertension drugs by lowering the price of volume procurement, accelerating the inclusion in the medical insurance directory and encouraging the research and development of new drugs. In general, the market of generic drugs in recent years has a huge impact, but there are still unmet clinical needs in this field. The successful approval of Aprocitentan marks the dawn of the development of new drugs in the field of hypertension treatment, and the future is foreseeable.




