On April 26,2024, the United States Food and Drug Administration (FDA) announced that accept Pfizer submitted Beqvez (active ingredient: Fidanacogene elaparvovec-dzkt) new drug marketing application for the treatment of 18 years or above moderate to severe hemophilia B adult patients, who do not with targeted adeno-associated virus (AAV) serotype Rh 74 subtype neutralizing antibodies.
Figure 1: Pfizer has received FDA approval for hemophilia B therapy
Beqvez Therapy is a gene therapy that allows a long-term, stable expression of bioactive coagulation factor IX (FIX) in humans, with sustained efficacy in patients with hemophilia. The PDUFA of this therapy is set to the second quarter of 2024. Beqvez Is priced at $3.5 million, the same with the previously approved hemophilia B gene therapy Hemgenix (active ingredient: Etranacogene dezaparvovec).

Figure 2: Two hemophilia B gene therapies currently on the market
Hemophilia profile and its intrinsic mechanisms
Hemophilia is an X-linked recessive inherited hemorrhagic disorder caused by F / F production disorders caused by mutations in a gene encoding the coagulation factor / (F / F). Hemophilia A is caused by F deficiency, and hemophilia B is due to F deficiency. According to the World Hemophilia Consortium, about 800,000 people worldwide have haemophilia, with more than 38,000 people living with haemophilia B. The registration of hemophilia initiated by the Hematology Hospital of the Chinese Academy of Medical Sciences shows that the number of hemophilia patients in China has reached more than 34,000 cases.

Figure 3: Hemophilia inheritance (a father with hemophilia will pass the X chromosome mutation to his daughter while the son is not affected; a mother with hemophilia will pass the X chromosome mutation equally to his daughter and son)
At present, the treatment of hemophilia is mainly through lifelong injection of exogenous coagulation factors to make up for their own deficiency, but due to the short half-life of coagulation factors and the need for frequent intravenous administration, it greatly affects patients' compliance and quality of life. Gene therapy provides a new and effective means for patients with hemophilia, which can introduce the F / F gene into the target cells through the vector, thus producing endogenous F / F, and then produce continuous efficacy in patients with hemophilia, and greatly improve patient compliance and adaptability. Vectors used in gene therapy include non-viral vector and viral vectors, among which adeno-associated viral vector (AAV) is the preferred vector for hemophilia gene therapy, characterized by low immunogenicity and high safety.
Beqvez Is a gene therapy based on an adeno-associated virus (AAV) vector that uses the AAV Rh74var envelope to deliver a highly active variant of the functional human FIX gene. The AAV Rh74var envelope is able to transduce hepatocytes, which are natural sites for FIX synthesis, and gene expression is driven by liver-specific promoters to generate tissue-specific expression. Thus, Beqvez helps hemophilia B patients to restore FIX procoagulant activity and hemostatic potential, thereby limiting bleeding and demand for exogenous FIX therapy.
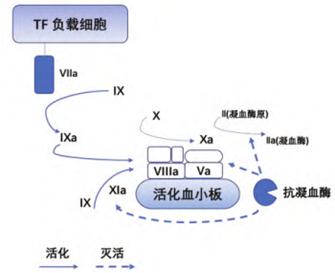
Figure 4: Interactions between the blood coagulation factors
Beqvez Initially developed by Spark Therapeutics (which has been acquired by Roche), in December 2014, Pfizer reached a partnership with Spark to acquire a global stake in the product for a total transaction value of US $280 million.
Beqvez The basis for the approved listing
This FDA approval is based on the results of a pivotal phase 3 clinical trial (NCT03861273) that began implementation on 29 July 2019 and is expected to be completed in 2030. This trial evaluated Beqvez, the efficacy and safety of adult male patients with moderate to severe hemophilia B, recruited 45 adult male patients with moderate to severe hemophilia B, all patients received FIX prophylaxis in routine care before entering clinical studies, and completed a prospective pilot study of at least 6 months to collect baseline data. The primary objective of the study is to assess the annual bleeding rate (ABR) in participants receiving gene therapy versus FIX prophylaxis alternative therapy.
The enrolled patients received 510 once11With intravenous infusion of vg / kg of Beqvez and entered a 6-year follow-up period, out of 45 patients, 41 completed at least 15 months of follow-up. The table below summarizes the results of this clinical trial, showing that the baseline mean ABR before Beqvez was 4.5 bleeding / year (95% CI: 1.9,7.2), the mean ABR after Beqvez was 2.5 bleeding / year (95% CI: 1.0,3.9), and the number of bleeding after Beqvez decreased significantly, meeting the criteria for study success and reaching its primary endpoint. Compared with FIX prophylactic regimen, the annual bleeding rate (ABR) after Beqvez infusion. According to the BENEGENE-2 positive data published by Pfizer in December 2022, this gene therapy has reduced the ABR of patients by 71% and reduced the annual rate of blood coagulation factor infusion by 92%.
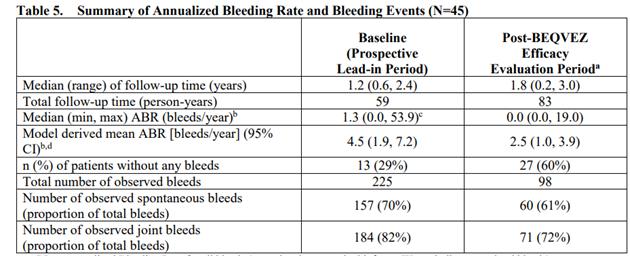
Figure 5: Beqvez, Key clinical trial results
Beqvez In the ClinicalTrials. Gohas registered four clinical trials related to hemophilia B, completed safety studies and dose-dependent studies on Beqvez, and efficacy studies are continuing, and currently available data show non-inferiority and superiority compared with FIX prophylactic treatment regimen. The following figure summarizes the four clinical studies of Beqvez.
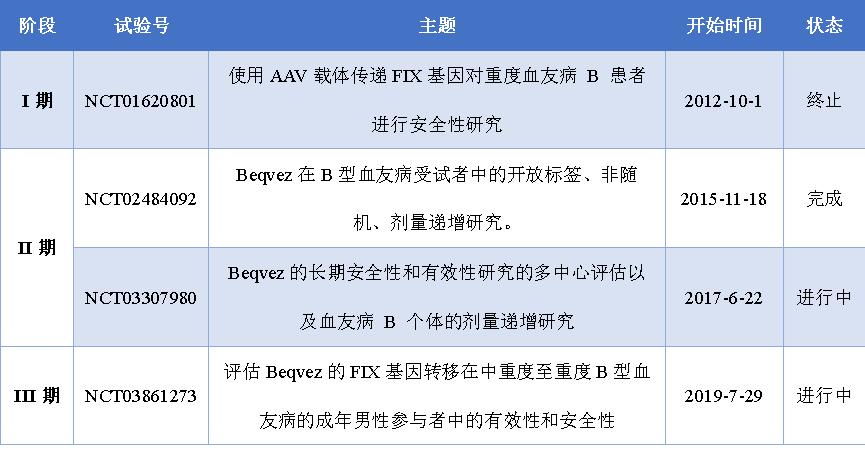
Figure 6: Beqvez in the ClinicalTrials. Clinical trials of the gov registry
sum up
Beqvez Is the second hemophilia B gene therapy approved by FDA after Hemgenix. With an intravenous infusion, it can stably express biologically active coagulation factor IX (FIIX) for a long time in the human body. Compared with the traditional prophylactic infusion of FIX, it greatly improves patient compliance and adaptability. Such effective and innovative therapies will revolutionize eligible patients, but very few patients can afford the high $3.5 million price.
reference material:
[1]FDA official website: https: / / www.fda.gov/
[2]Pfizer's official website: https: / / www.pfizer.com/
[3]https://labeling.pfizer.com/ShowLabeling.aspx?id=20452
[4]Drug Bank Official website: https: / / go.drugbank.com/




