On April 30,2024, the State Medical Products Administration (NMPA) approved the enostat tablets (trade name: Jingjuda) declared by Taizhou Eteng Jingang Pharmaceutical. The drug is used in combination with aromatase inhibitors to treat patients with locally advanced or metastatic breast cancer who are positive for hormone receptor (HR) and human epidermal growth factor receptor-2 (HER 2) negative and have relapsed or progressed with endocrine therapy.

Figure 1: NMPA announces approval of entinostat
Breast cancer (BC) is the most common malignancy in women, accounting for nearly one-third of all newly diagnosed cases. HR-positive / HER 2-negative breast cancer (HR + BC) is the most common breast cancer and is characterized by estrogen or progesterone receptor (ER / PR) positive HER 2-negative malignant cells, accounting for approximately 60-70% of all BC. For such tumors, endocrine therapy is the main means of treatment, and antiestrogen therapy is the first targeted therapy approved for HR + BC therapy. However, due to the heterogeneity of the disease, HR + BC has developed resistance to endocrine drugs, with 15% – 20% of tumors having intrinsic resistance and 30% – 40% of tumors gaining resistance over time.
Mechanistically, altered gene expression caused by epigenetic modifications leads to both primary and secondary resistance to endocrine therapies. Histone modifications have an important role in the epigenetic modification mechanism. The modification of histones mainly includes histone acetylation, a process that is regulated by two functionally mutually antagonistic enzymes: histone acetyltransferase (HAT) and histone deacetylase (HDAC). As shown in the figure below, HAT regulates histone acetylation, which facilitates the dissociation of DNA from histone octamers, so that various transcription factors can specifically bind to DNA binding sites and activate gene transcription, while HDAC plays the opposite role.

Figure 2: Regulation of histones by HAT and HDAC
In cancer cells, overexpression of HDAC leads to enhanced deacetylation against the expression of specific genes, including some tumor suppressor genes. Histone deacetylase inhibitors (HDACi) can induce antitumor immune responses and reverse the epigenetic modifications that lead to resistance to endocrine therapies.
In recent years, with the increasing international research on the relationship between HDAC and cancer development, HDACi has become a research hotspot of anti-tumor drugs at home and abroad. HDACi can reduce the proportion of histone deacetylation in tumor cells by inhibiting the activity of HDAC, causing cell cycle arrest, pro-differentiation, and induction of apoptosis. As a new generation of targeted antitumor drugs, HDACi has low toxicity and highly efficient antitumor effects compared with traditional drugs. HDACi According to its different chemical structure, it can be divided into four categories, namely, hydroxamic acids, cyclic peptides, fatty acids, and benzamide.
Enterostat tablet is an inhibitor of benzamide histone deacetylase (HDAC), which can selectively inhibit class I and class IV HDACs, inhibit cell proliferation, promote terminal differentiation and / or induce apoptosis, and play antitumor effects. Entinostat has a long half-life in the body (h1/2=33-150h), this unique feature distinguishes him from other drugs of the same type, and entinostat can upregulate the expression of aromatase and restore cell sensitivity to non-steroidal aromatase inhibitors (NSAIs), so combining entinostat with aromatase inhibitors will have a synergistic therapeutic effect. The drug offers a new treatment option for patients with hormone receptor (HR) -positive and human epidermal growth factor receptor-2 (HER-2) -negative locally advanced or metastatic breast cancer.
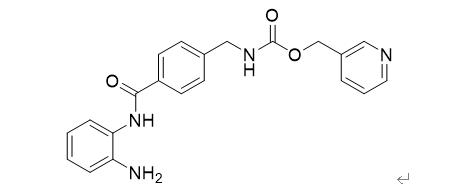
Figure 3: Entinostat structure formula
This NMPA approval is based on a randomized, double-blind, controlled phase III clinical study (NCT03538171) in patients with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER-2) negative advanced breast cancer. This study was implemented on 15 May 2018 and reached the preset primary study endpoint in July 2021. A total of 354 patients were randomized to enostat (n = 235) and placebo (n = 119).
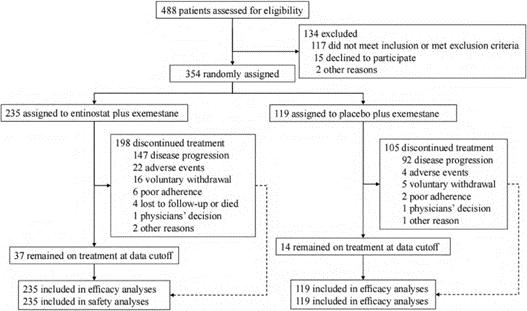
Figure 4: NCT03538171 Clinical study flow chart
The results showed that the progression-free survival (PFS) was significantly longer than the placebo group by 6.32 months (95%CI, 5.30-9.11) and 3.72 months (95%CI, 1.91 – 5.49) versus placebo group; the risk of disease progression or death was 24% compared with placebo, and overall survival decreased by 16.3%, and the benefit from premenopausal or perimenopausal patients was consistent with postmenopausal patients. The study safety data were consistent with the known safety characteristics of the drug. These results suggest that the aromatase inhibitor exemestane combined with entinostat is significantly improved in HR + / HER 2 ABC patients with prior endocrine therapy.
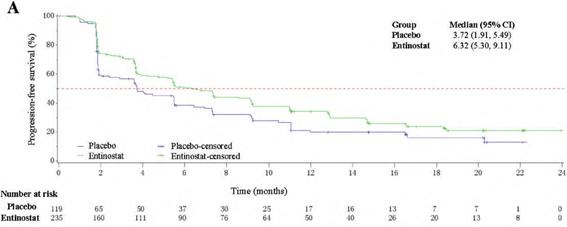
Figure 5: Results of progression-free survival (PFS) in the entemostat versus placebo
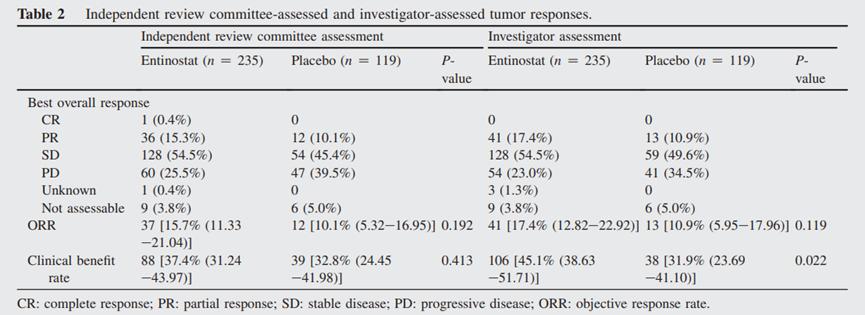
Figure 6: Clinical benefit rates in the entemostat and placebo groups
So far, entinostat has been used in ClinicalTrials.gov registered 67 clinical trials, a total of 77 clinical trials worldwide ongoing or completed, these clinical trials mainly involves the following indications: breast cancer, lymphoma, advanced renal cell cancer, metastatic non-small cell lung cancer, pancreatic cancer, etc., the entostat effectiveness, safety, drug interaction, pharmacokinetics and pharmacodynamics drug properties made a comprehensive study. The approval of Enmesat in China is undoubtedly a positive signal to other projects under research. The following summarizes important clinical studies of entinostat in the treatment of HR positive / HER 2 negative breast cancer.
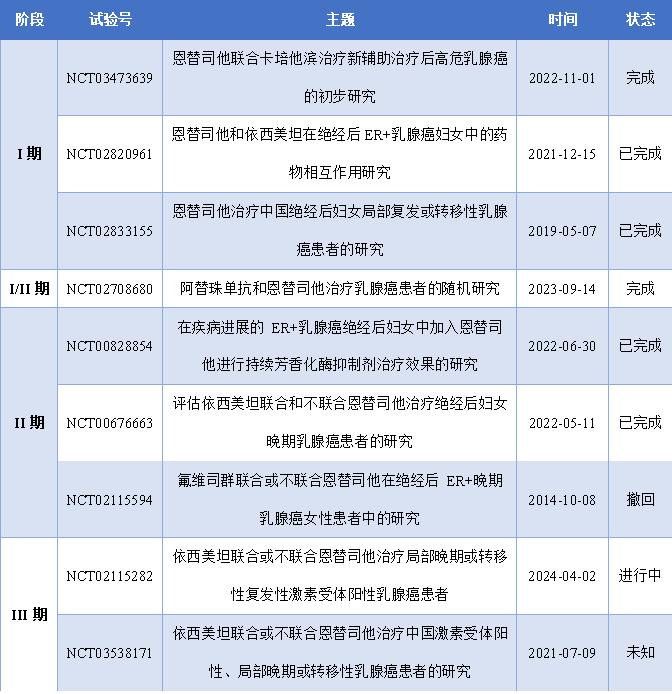
Figure 7: Enostat important clinical studies on breast cancer
In recent years, HDAC inhibitors have become a hot spot in the research and development of anti-tumor drugs at home and abroad. According to statistics, there are 330 HDAC targets under development, involving 1,609 related clinical trials and 27,773 patents. With its unique characteristics of long half-life, entinostat tablets greatly improves patient compliance and reduces the cost of medication, making it further differentiated from other HDAC inhibitors, with the potential of "best-in-class". Look forward to more good news about the approval of new indications for entinostat in the future.
reference material:
[1] NMPA official website: https: / / www.nmpa.gov.cn
[2]Xu B, Zhang Q, Hu X, et al.Entinostat, a class I selective histone deacetylase inhibitor, plus exemestane for Chinese patients with hormone receptor-positive advanced breast cancer: A multicenter, randomized, double-blind, placebo-controlled, phase 3 trial.Acta Pharm Sin B.2023;13(5):2250-2258.
[3]Drugbank Official website: https: / / go.drugbank.com/
[4]Pubchem Official website: https: / / pubchem.ncbi.nlm.nih.gov/




