On April 26,2024, The US Food and Drug Administration (FDA) announced the approval of X4 Pharmaceuticals's new molecular entity, Xolremdi (active ingredient: Mavorixafor), Xolremdi Is a selective CXC chemokine receptor 4 (CXCR 4) antagonist, For patients age 12 years and older with WHIM syndrome (warts, hypogammaglobulinemia, infection and bone marrow granulocydeficiency), To increase the number of mature neutrophils and lymphocytes in the circulation. Xolremdi, Is the first therapy used specifically used in patients with WHIM syndrome.

Figure 1: The FDA has approved the Xolremdi for marketing
WHIM syndrome is a rare congenital immunodeficiency disease characterized by human papillomavirus (HPV) warts, hypogammaglobulinemia, bacterial infection and ineffective generative chronic paraglocytic deficiency. Patients with WHIM syndrome are characterized by neutrophil and lymphopenia in the blood and experience severe and / or frequent infections, being an autosomal dominant disorder. It is estimated that approximately 1 in 5 million live births, and about 60 cases have been reported in the medical literature. At least 1,000 people in the United States have been diagnosed with WHIM syndrome, and in addition, cases of WHIM syndrome have been reported in Europe, Japan and China, in both men and women.
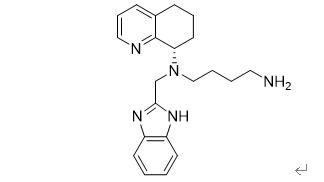
Figure 2: The Xolremdi structure
Current stage studies have demonstrated that WHIM syndrome is caused by heterozygous mutations in the CXCR 4 gene, resulting in impaired mobilization of leukocytes from the bone marrow into the peripheral circulation. CXCR 4 is expressed on most of the mature leukocyte subtypes, as well as on hematopoietic progenitor cells, with lymphocyte chemotactic properties. The ligand of CXCR 4, the bone marrow stromal cell-derived factor (SDF-1, also known as CXCL12), plays an important role in hematopoietic stem cell homing and dormancy.
When CXCL12 binds to CXCR 4, signal transduction activates downstream effectors such as AKT and extracellular signal-regulated kinases (Erk) to trigger cell retention through calcium ion flow. CXCR 4 mutation in WHIM patients enhanced its activity, resulting in delayed release of mature neutrophils from the bone marrow and reduction of peripheral blood leukocytes, while mature leukocytes remaining in the bone marrow further apoptosis, thus reducing neutrophils and lymphocytes in the blood of the patients.
Thus Xolremdi is an orally bioactive CXCR 4 antagonist that blocks the binding of the inhibitory ligand CXCL12 and its receptor CXCR 4, increasing the number of mature neutrophils and lymphocytes in circulation and improving symptoms in WHIM patients.
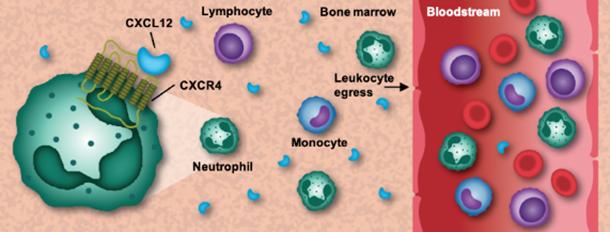
Figure 3: The CXCR4 pathway regulates the mobilization of leukocytes from the bone marrow to the peripheral blood
FDA approval of Xolremdi was based on the results of a pivotal phase 4WHIM 3 clinical trial initiated on 24 October 2019, as a global, randomized, double-blind, placebo-controlled, 52-week multicenter study evaluating the efficacy and safety of Xolremdi in 31 patients diagnosed with WHIM syndrome aged 12 years and older.
Thirty-one patients were randomized in a 1:1 ratio to receive either placebo (17) or Xolremdi (14) once daily for 52 weeks. Xolremdi The efficacy of treating patients with WHIM syndrome is based on improvement in absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and decreases in infection. In the 4 WHIM trial, Xolremdi treatment showed longer absolute neutrophil count (TAT-ANC) above the threshold (500 cells / microliter) than placebo, and longer absolute lymphocyte count (TAT-ALC) above the threshold (1000 cells / microl) than placebo. In terms of safety, the annual infection rate was 60% lower in patients receiving Xolremdi as compared to patients receiving placebo.4 The WHIM trial reached its primary and key secondary endpoints and was generally well tolerated in the trial, reporting no serious adverse events related to treatment or discontinuation due to a safety event.

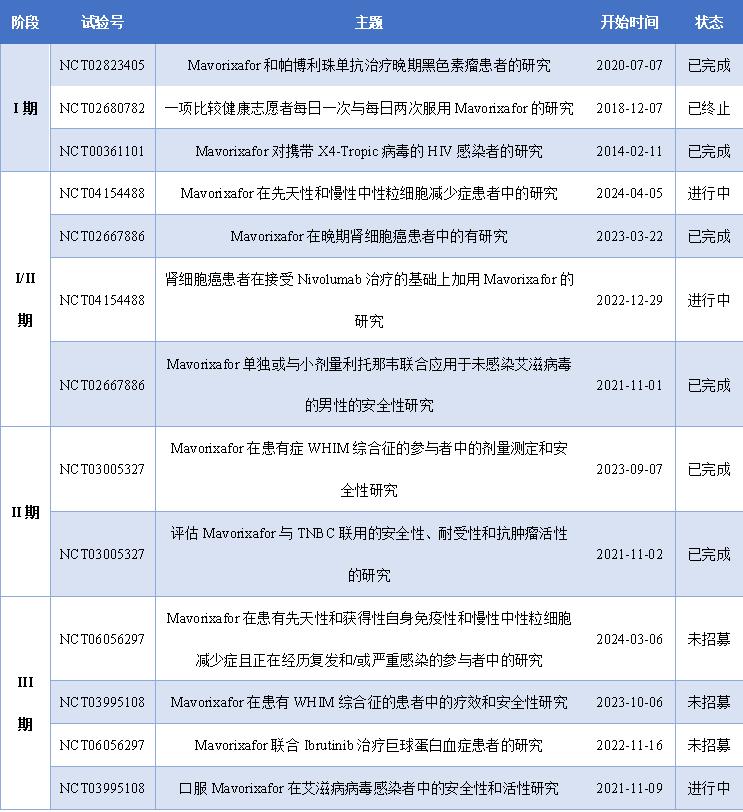
Figure 4: Xolremdi Clinical trial results
X4 Pharmaceuticals With the support of the results of the global pivotal phase 4WHIM 3 clinical trial, the Company submitted to the FDA a new drug marketing application (NDA) for the treatment of WHIM syndrome with Mavorixafor on September 5,2023, and announced on October 31,2023 that the FDA has received the priority review for Mavorixafor for WHIM syndrome. The following below summarizes the clinical studies Mavorixafor underwent from development to marketing.
Previously, treatment for patients with WHIM syndrome had focused on symptom control rather than the root cause of the disease — dysfunction of the CXCR 4 pathway. Xolremdi Is a therapy designed to address the problem of the dysregulation of CXCR 4 pathway signaling, and the approval of this targeted therapeutic approach has been shown to increase the absolute number of neutrophils and lymphocytes and enhance resistance to infection in WHIM patients.
reference material:
[1] FDA official website: https: / / www.fda.gov/
[2]X4 Pharmaceuticals Official website: https: / / www.x4pharma.com/
[3] Song Zeliang, Shi Xiaodong. Progress in the study of WHIM syndrome [J]. China Medical Journal, 2016,51 (04): 28-30.
[4]Drug Bank Official website: https: / / go.drugbank.com/




