On May 15, Bristol-Myers Squibb (BMS) announced that the Food and Drug Administration (FDA) has accelerated the approval of autologous CAR-T cell therapy Breyanzi ® (lisocabtagene maraleucel; liso-cel) targeting CD19 antigen for adult patients with relapsed or refractory follicular lymphoma (FL) who have previously received two or more systemic therapies. The indication is approved based on response rate and response duration, and continued approval of the indication may depend on the validation and description of the clinical efficacy of the confirmatory trial.

This is the fourth time that the Breyanzi has received FDA approval for a new indication.
- In February 2021, Breyanzi was first approved in the United States for adults with relapsed or refractory large B cell lymphoma (LBCL) treated with two or more systemic therapies, including diffuse large B cell lymphoma (DLBCL), high-grade B-cell lymphoma, primary mediastinal large B cell lymphoma, and grade 3B FL.
- On 24 June 2022, Breyanzi was approved by the FDA for second-line treatment of adult B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and grade 3B FL.
- On 15 March 2024, Breyanzi was FDA approved for the treatment of adult patients with relapsed / refractory chronic lymphocytic leukemia (R / RCLL) or small lymphocytic lymphoma (SLL) who had previously received at least two lines of treatment, including BTK inhibitors and BCL-2 inhibitors.
- At the end of this month, the FDA's regulatory decision will also allow the Breyanzi application to treat adult patients with mantle cell lymphoma (MCL).
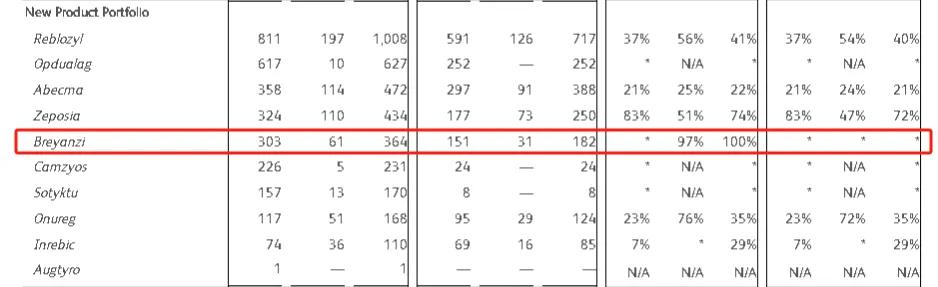
Since launch, Q1Breyanzi 2024 sales were $107 million, up 51% year on year; Breyanzi in 2023 sales were $364 million, up 100% year on year; Breyanzi in $182 million in 2022 to $87 million in 2021. In the three years since its launch, it has sold $740 million (about 5.347 billion yuan).
FL has long been considered as an incurable disease, where patients often relapse after first-line therapy, and the prognosis is worsened after each relapse. Despite advances in treatment, there is still a need for more options to provide a treatment-free interval and a durable, complete response.
This accelerated approval is based on the positive results of the phase 2 clinical trial TRANSCEND FL. TRANSCEND FL (NCT04245839) To determine the efficacy and safety of Breyanzi in patients with relapsed or refractory non-Hodgkin B cell lymphoma (including follicular lymphoma). The primary outcome measure is the overall response rate, and secondary outcome measures include complete response rate, duration of response, progression-free survival, and safety.
Results showed that the overall response rate (ORR) was 95.7% in patients treated on third line with Breyanzi in the primary efficacy analysis group (n=94) (95% CI:89.5-98.8). The complete response rate (CR) was 73.4% (95% CI:63.3-82.0). The median duration of response (DOR) was not reached (95% CI:18.04-NR), with 80.9% of responders remaining at 12 months and 77.1% at 18 months.
The results of the TRANSCEND FL main analysis presented at the International Conference on Malignant Lymphoma 2023 showed that the ORR of evaluable patients (n=101) was 97% (95% CI:91.6-99.4; unilateral p <0.0001) and 94% achieved CR (95% CI:87.5-97.8; unilateral p <0.0001).

Breyanzi With a stable safety profile, 53% of patients developed cytokine release syndrome (CRS) of any grade, and 4% had CRS greater than grade 3. The median onset time was 5 days (range: 1-63 days). Neurological events (NEs) of any level occurred in 31% of the patients, with 10% having NEs of a level> 3. The median time to the occurrence of NEs was 8 days (range: 1-63 days).
Bryan Campbell, senior vice president and business head of cell therapy at Bristol-Myers, said, " Breyanzi is the cornerstone of our cell therapy portfolio and provides differentiated treatment options for a variety of B cell malignancies. Today, Breyanzi is approved for the treatment of relapsed or refractory FL, providing an option for a single infusion, and its safety profile allows it to be administered and monitored in the inpatient and outpatient setting in a growing number of certified treatment centers in the United States.”




