In recent years, the number of published articles or comments on "3D cell culture" has increased rapidly, and the organoid technology derived from 3D cell culture has also become a powerful tool for researchers.
3D cell culture, also known as three-dimensional cell culture, refers to the joint culture of animal cells with scaffold materials with three-dimensional structure, so that cells can grow, multiply and migrate in three-dimensional space, forming three-dimensional cell-cell or cell-carrier complex, so as to better simulate the growth environment of cells in the body.
Suitable for: molecular and cell biological analysis, between the animal and cell levels, widely used in functional tissue induction, disease model establishment, drug screening, anti-inflammatory testing, clinical research and other aspects of the research.
Organoids is a microorgan with three-dimensional structure cultured in vitro environment. It has complex structures similar to real organs and can partially simulate the physiological functions of tissues or organs of origin (stem cells, tumor tissue, patient source, etc.). Up to now, more than 10 different tissues, disease models and simulated development of organoids have been developed. As a major technological breakthrough, organoids have been recognized as an important tool in the field of biological science research, and have an important clinical application value.
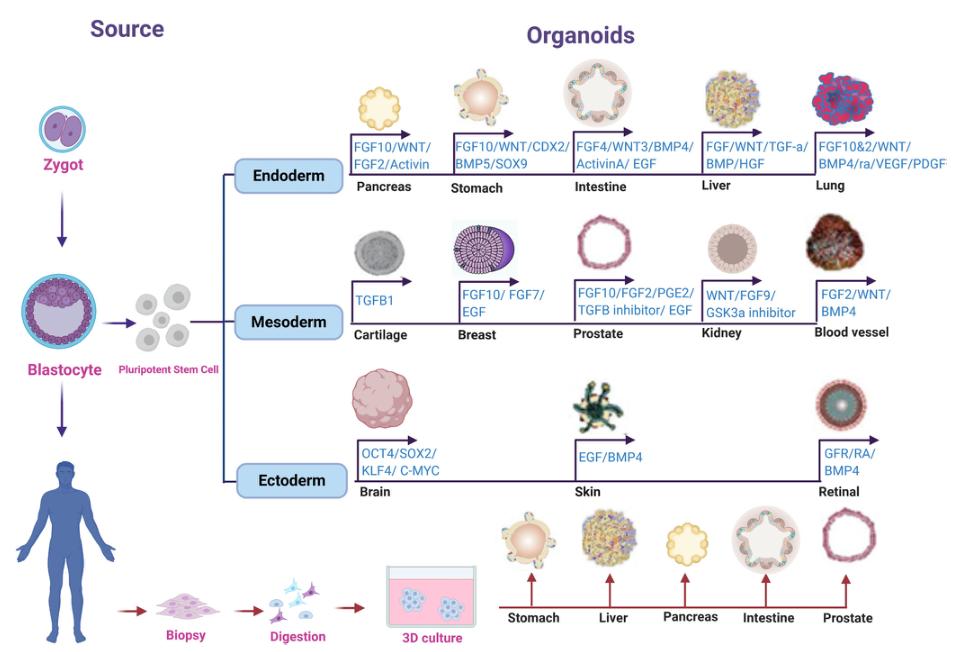
Ref.: Organoid technology Current standing and future perspectives
From the perspective of the definition and source of organoids, it is to culture 3D cells of pluripotent stem cells or patient-derived tumor tissue, so as to form the self-organization of the corresponding organs, and have the ability of self-renewal and self-organization, so it is also called "the organ in a flat dish". Organoids belong to 3D cell cultures that contain organ-specific cell types and can exhibit the spatial organization of organs and certain functions of replicating organs. Organoids recapitulate a physiologically highly relevant system where complex problems can be investigated in three-dimensional systems. For example, disease onset, tissue regeneration, and interactions between organs.
Although these organoids, formed by a large number of cells, can simulate the internal structure of real organs in many ways, some structural characteristics closely related to the function and development of real organs are still inaccessible, such as the lack of vascular system, which is an important structure to obtain energy in the growth and development of human organs. Therefore, until far, organoids cannot be called a "miniature version" of real organs, and are still miniature and simple organ models.
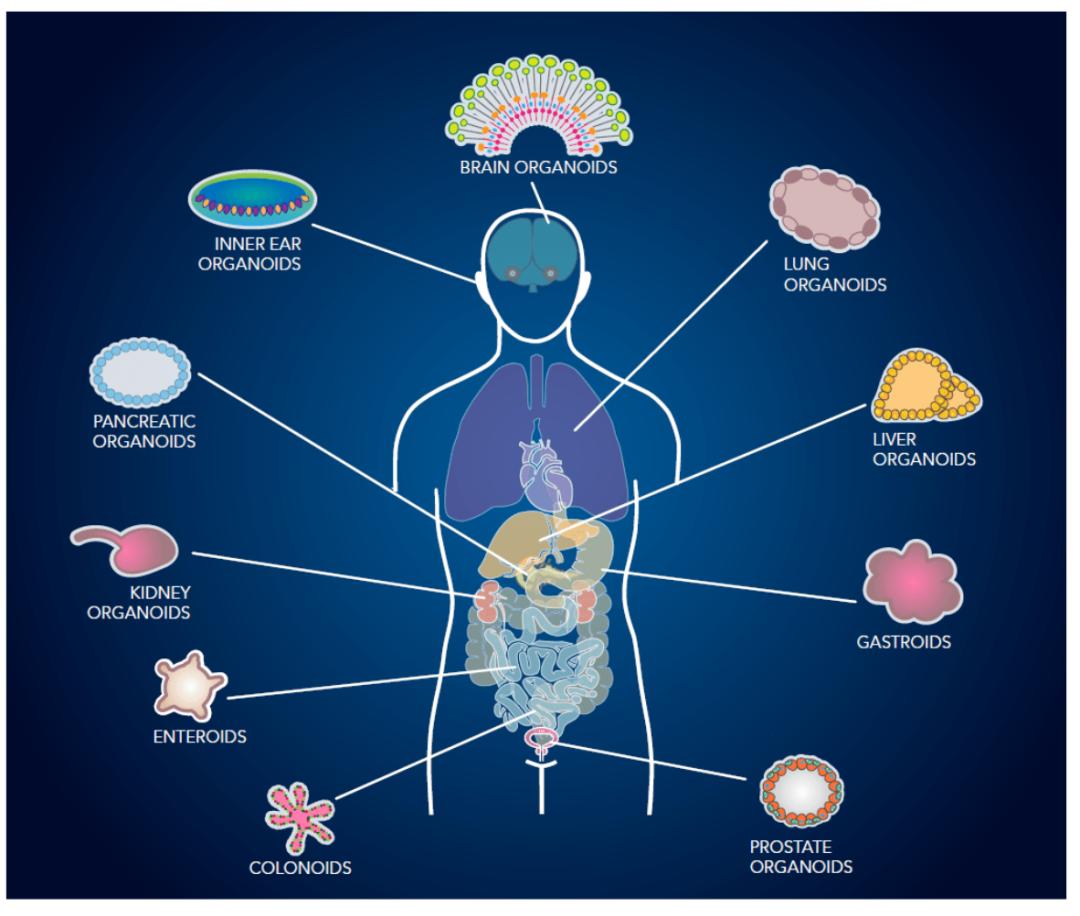
At present, the culture of organoids mainly refers to epithelial cell organs, such as digestive tract epithelial cells, mammary gland epithelial cells, skin epithelial cells, alveolar epithelial cells, etc. Most of the organoids only epithelial cells, do not contain fibroblasts, immune cells, vascular cells and other surrounding stromal cells.
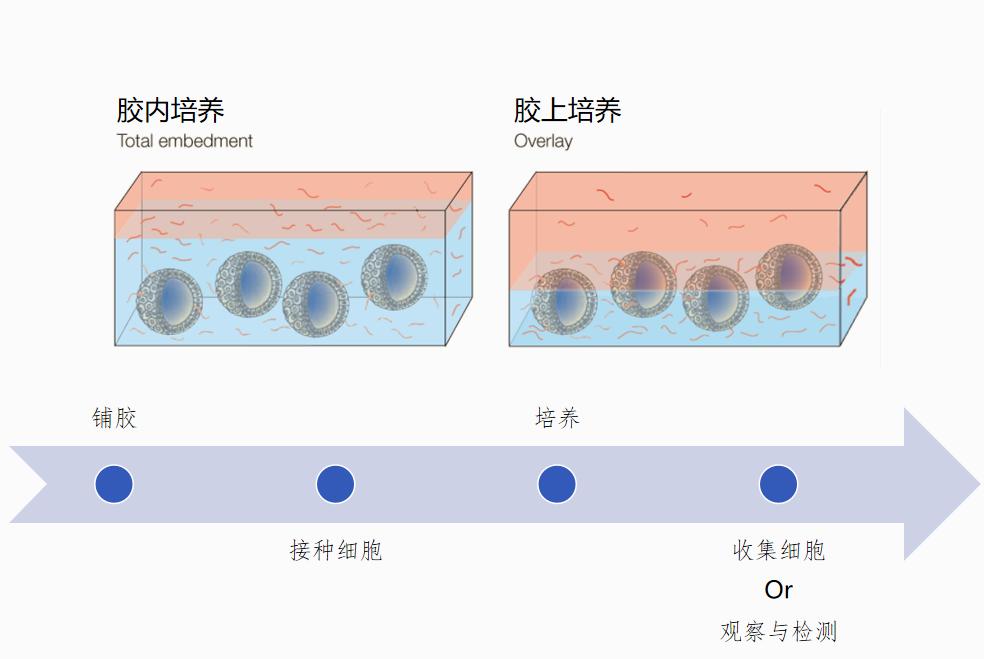
How the organoid culture is performed
What is the difference between conventional cell culture?
How to achieve a 3 D cell culture:
Cultivation: As a platform for tissue engineering, the scaffold not only provides a framework for cell growth to form specific tissue or organ shapes; but also, as one of the extracellular matrix components, it is a medium of cell-cell signaling and interaction, and a bioactive agent necessary for cell growth.
3 D cultivation of magnetic suspension: Rice University and the University of Texas M.D. Anderson Researchers at the Cancer Center have developed a biological assembler (bioassembler). This system uses a magnetic force to suspend the cells and cause them to grow into a three-dimensional shape. It is especially suitable for cultivating liver and heart, a solid organ with large cell density.
3 D hydrogel culture: the cells are grown in a certain extracellular matrix, and the extracellular matrix (extracellularmatrix, ECM) protein acts as a growth scaffold. Jeffrey Morgan, a biological engineer at Brown University, has developed a three-dimensional petri dish using agarose. The melted agarose was made into a miniature culture plate (micromoulds), and after the agarose had solidified, the agarose material was placed into a regular culture plate. Cells and media are added to the culture plates, and due to gravity, the cells are deposited into the agarose culture plates and clustered together with each other, forming spherical multicellular masses.
1) Three-dimensional structure: Organoid cultures are conducted in 3D structures to better simulate the spatial arrangement and interaction of cells in the body, while conventional cell culture is usually in a flat 2D environment.
2) Diversity of cell types: Organoids can contain multiple types of cells to better reproduce the complexity of the organ, while traditional cell culture usually involves a single cell type.
3) Microenvironment simulation: Organoid culture puts more emphasis on the simulation of the body microenvironment, including cell-cell interactions and matrix composition, while 2D culture considers less about these factors.
4) Usage and application: Organoid culture is suitable for studying complex cell interactions, disease models, drug screening, etc., while conventional 2D cell culture is more used for basic cell biology research.
5) Technical complexity and cost: Organoid culture techniques are usually more complex and more costly, and require professional technology and materials.
There are many different protocols and methods available in 3D cell culture. Here are some common 3D cell culture protocols or methods:
Porous scaffold method: porous materials (such as sponge, gel, or polymer scaffolds) are used to provide a three-dimensional support structure for cell growth. This approach can control cell distribution and growth by regulating the porosity and shape of the scaffold.
The porous scaffold method is a commonly used method for preparing the 3D cell culture matrix. This method constructs a three-dimensional matrix with a fine pore structure on a scaffold within which cells can colonize and grow. Porous scaffolds can be prepared by biodegradable or non-biodegradable materials with good biocompatibility and tissue similarity, thus providing the support needed for cell growth and a suitable microenvironment.
Self-assembly method: using the cell itself adhesion and interaction forces to form a three-dimensional structure without external support. This can be achieved by adjusting the cell density, cell type, and culture conditions.
Self-assembly is a technique that uses the spontaneous assembly of various cells to form tissue structures. In this approach, cells assemble spontaneously into tissues with a specific structure and function through adhesion molecules or specific cell-cell interactions. This approach allows for the preparation of complex multicellular systems and the reconstruction of tissue structures and physiological functions in vitro.
3D printing method: Use 3D printing technology to print and assemble cells and scaffold materials according to the designed structure and shape. This approach produces complex and customized 3D cell constructs.
3D printing is a technique that prints cells and biomaterials layer by layer into a 3D structure. By using a 3D printer, biological materials and cells can be printed layer by layer according to preset designs and models, resulting in a tissue or organ model with complex structures. This approach allows precise control of cell distribution and shape of the scaffold for customized 3D cell culture.
Suspension culture method: the cells were cultured in suspension in the medium without support. This method allows free cells to form three-dimensional aggregates such as spheres or clumps.
The suspension culture method is a method for suspending cells in culture medium. In this approach, cells can be freely suspended in a liquid environment to form 3D structures by cell-autonomous self-assembly or microenvironmental regulation. Suspension culture method is often used to culture complex multicellular systems, such as tumor spheroids.
Microfluidic technology: Using the special texture and structure of microfluidic devices and microfluidic chips to control the location and growth of cells in 3 D space. This approach can mimic the in vivo microenvironment, enabling a more precise and controlled cell culture.
Microfluidic technology is a method of using micromachining techniques for precise cell and fluid control using microfluidic channels and microvalves. Precise control and adjustment of the cell and culture environment can be achieved through microfluidic technology, simulating the complex internal environment. This approach is often used to study cell-cell interactions, cell migration and tissue remodeling.
Edenation: The cells are cultured on a material or substrate with special properties and then the cell structures are stripped down using specific stripping techniques. This method produces thinner 3D cell structures.
Edenation is a method of stripping or removing cells from the outer layer of a tissue or organ, and then reassembling the stripped cells into 3D structures. Exdenation allows to reshape the tissue morphology and structure and study the ability of redifferentiation and cell-self-assembly.
Tissue engineering: using the principles and techniques of tissue engineering to combine cells and biological materials to form 3D structures with similar tissue structures and functions. This approach allows to simulate and reconstruct complex tissues and organs through a combined culture of multiple tissues.
Tissue engineering method is to combine biological materials with cells to construct tissue structures with biological functions. Usually, tissue engineering uses biomaterials as scaffolds or carriers to promote cell attachment, proliferation, and differentiation. Through rational selection and design of biomaterials, the environmental and stimulating effects of cell culture can be improved and tissue regeneration and repair can be promoted.




