Since the approval of estradiol patch on January 1,1997, a total of 628 approval numbers have been approved, involving 114 active ingredients (including traditional Chinese medicine). Generally speaking, the number of patches marketed is not large, which belongs to the relatively partial dosage form. In recent years, with the implementation of consistency evaluation policy and centralized drug procurement policy, the country has paid more and more attention to the differentiation of drugs and paid attention to the rational application of drugs, which has promoted the research and development heat of domestic enterprises. How to quickly start the application into this field is a problem worth thinking about.
Generic drug approval of the patch
At present, there are 72 valid approvals for chemical drug listed in China, involving 36 active ingredients, and there are not many varieties available for imitation. Since the implementation of "quantity procurement" related policy, cancel the drug middlemen, drug sales directly determined by the purchase quantity, so the enterprise in the project more attention to the degree of competition, and the original research whether to enter the domestic market, in a large degree also affects the development of generic drugs, according to the above situation, divided into the following two categories are analyzed.
1. Domestic unlisted generic drugs, including the original research products
This kind of product is the least competitive, once the imitation market almost monopolize the market, but there is also some difficulty in imitation:
In terms of information, because the original research products are not listed in China, it is difficult for general enterprises to obtain comprehensive information, and it is difficult to develop. For example, the fibrin patch for the treatment of blood coagulation abnormalities was developed by EthiconBioSurgery company, due to the less information of fibrin, it is difficult to copy;
Clinically, there are many uncertainties in clinical outcomes due to ethnic differences (for white and black people);
In terms of market, as the original research company pays more and more attention to the domestic market, the better products sold in foreign countries will be considered listed in China, and the market potential of the remaining products not listed in China is worth discussing;
Drug habits, foreign patch on a long time treatment field, many areas in the domestic no patch products, not acceptable to domestic, such as for the treatment of migraine of succinate schumaptan patch and used for the treatment of mental classification of asenapapine patch, in domestic manufacturers to declare, competition is smaller, but the two treatment areas in the domestic not patch products, its medication habits are recognized still need further discussion.
Of course, according to the strength of the enterprise itself and the specific characteristics of the product analysis, this kind of product once successfully approved by the project, the income is not small.
2. The original research institute is listed in China, but there are no listed products of local enterprises
The original research product has been listed in China. To some extent, the original research company has confirmed the market value of the product in China, and has certain advantages in terms of market capacity and clinical practice. At the same time, its information is more comprehensive and transparent, so it is not difficult to develop. This is an ideal product for small and medium-sized enterprises. Of the 36 active ingredients already on the market, 9 varieties meet the current conditions, specifically as follows:
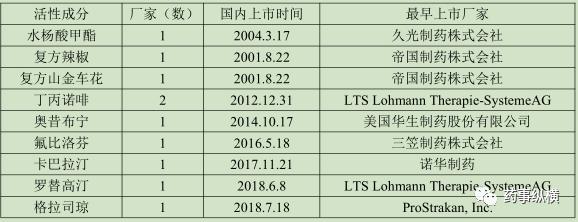
These 9 active ingredients are all listed in China, among which oxxibutin, carbabatine, rotigotin, granissetron, flurbiprofen and other products are listed in recent years, no local enterprises are listed, with a large space for imitation.
Oxybutynin was produced by Watson Laboratories, Inc. The company was imported into China in 2014. Its indications are overactive bladder, frequency, urgency, neurogenic incontinence, spontaneous detrusor instability and overnight enuresis, with a bioavailability of only 6% and a half-life of 2-3 hours. Compared with domestic marketed tablets, capsules and oral fluids, patches can prolong the treatment time of drugs through the skin; directly entering the blood through the skin can avoid the first pass effect and improve the bioavailability of drugs; finally, for children, patches are better than oral preparations in terms of compliance, so it is more in line with the current drug use and can comply with the current national policies and has great development value.
Kabbalatine patch (liss transdermal patch) is developed by novartis pharmaceutical, used for the treatment of mild to moderate Alzheimer's disease, the disease is mainly concentrated in the elderly, especially the elderly with alzheimer's disease in swallow more difficult, the development has significant improvement in medication, with agent also has the same advantage.
Buprenorphine was a partial agonist. The analgesic effect was stronger than pethidine, with a slow onset and a long duration. Most of the domestic patches are focused on analgesia and anti-inflammatory aspects. Similar fentanyl transdermal patches have been listed in China for many years and have been recognized by the society, so the listing of buprenorphine patches can also bring a certain market.
Fluorobiprofen for domestic excellent non-steroidal anti-inflammatory drugs, its curative effect and safety has been social recognition, its domestic injection, tablets, slow-release tablets, patch to a large extent bring the convenience of use, especially in the treatment of rheumatoid arthritis, the patch can play a better targeted effect, the variety and 600 million sales in 2017, has a very large market space.
Granisetron was originally developed by Beecham in the mid-80s. For radiation therapy, cytotoxin-induced nausea and vomiting. Meusea and vomiting caused by chemotherapy and radiotherapy has always been one of the serious side effects that worry doctors and patients, and setron drugs now occupy most of the market for antiemetic drugs. At present, granisetron has three dosage forms of capsule, oral collapse tablet and injection. It is not only convenient for patients to use, but also has significant effects in terms of toxic and side effects, and has a large market space.
Project analysis of improved new drug of patch
Patent through percutaneous absorption, drugs must have very good transdermal effect, and the dosage, indications, usage and dosage has strict requirements, plus in recent years for new clinical advantage look more and more heavy, no obvious clinical effect, simple change dosage form is not necessarily to get approval center, so the patch of new drug development is difficult. Since the reclassification of chemical drug registration in 2016, there are four patch new drugs, all of which are improved new drugs, as follows:

From the analysis of the above results, sufentanil transdermal patch has been approved for clinical practice, and its improvement degree has been recognized by the national Bureau. Sufentanil has strong analgesic effect, but the duration is short, it has a certain addiction, the development of patch can prolong its analgesic time, but also reduce certain side effects. This also reflects that, in addition to the advantages of the dosage form, the development of new drugs has improved in efficacy, toxicity and side effects, there is a better chance to be recognized.
AB001 has not obtained its active ingredient, lidocaine gel paste mainly adds new indications, and its review scale is mostly based on new indications, and the contribution of dosage form change is a small proportion.
Meloxicam diethylamide is further saline on the basis of meloxicam to form new compounds, but it does not change the efficacy of the original active ingredient, and can increase the water solubility of the original active ingredient after becoming salt. Whether there is a further role in the efficacy and toxicology has not found the relevant information. According to the review progress, the review of supplementary data was completed on September 18,2019, and it is unknown whether clinical approval or not.
To sum up, from the analysis of the four improved new drugs that have been declared, only changing the route of administration will not necessarily be recognized by the National Bureau, but with further improve in efficacy and toxicology, that is another matter. Of course, the above conclusions are only based on the analysis of the four improved new drugs that have been declared, with relatively few data, and none of them have achieved final production. The current conclusions may be biased, and enterprises need further analysis when approving the project.
epilogue
In recent years, patch more and more attention, many domestic enterprises will research and development direction in this aspect. However, in the technology, preparation equipment and auxiliary composition, for the development of new patch, not only the rationality of the dosage form, but also the clinical advantages. Finally, with the continuous implementation of relevant policies in China and the change of patients' concept of drug use, it is believed that the use of patches is more and more widely, so the development of patches is conducive to the development of enterprises.




