Early research into Excision BioTherapeutics, a biotech company developing CRISPR-based therapies to treat latent viral infections, using a CRISPR-based gene-edited therapy for HIV, has failed. Excision The Company's HIV program is the first CRISPR technology-based in vivo systemic therapy in the US to be evaluated in a clinical trial. The EBT-101-001 clinical study uses an open-label design to assess safety and tolerability as the primary endpoint.
The results of a phase I trial in five patients showed that CRISPR therapy with Excision was not effective in HIV suppression. Three patients were reported to develop viral rebound soon after ART discontinuation and required a return to conventional therapy.
Still, Excision says its approach shows signs of hope. One patient began to rebound 16 weeks after cessation of ART therapy. Typically, HIV levels will rise again after about four weeks. CRISPR Treatment also appeared to decrease the number of infected cells in this patient.

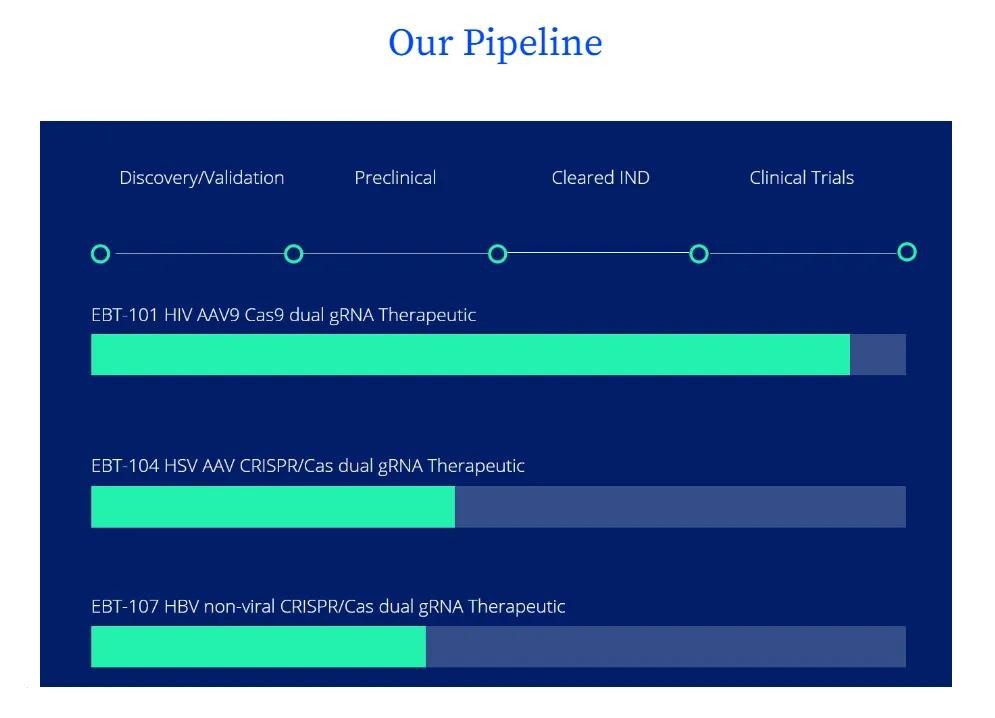
The main Excision company drug candidate is EBT-101, a CRISPR-based gene editor that removes HIV proviral DNA from all infected cells. The candidate is delivered through an AAV 9 vector using two guide RNA that target three target sites in the HIV genome, minimizing the possibility of viral escape. The company is positioning EBT-101 as a possible cure for chronic AIDS, and is "rapidly advancing clinical trials".
In October 2023, Excision released safety data for the first human phase I / II study of EBT-101, which showed that none of the three patients treated with gene editors had serious adverse events or dose-limiting toxicities. The study identified four mild toxicities that could be related to EBT-101, but all were alleviated without intervention. Early data also showed that EBT-101 was still detectable in the blood after four weeks of treatment.
In addition to targeting AIDS, Excision also uses its proprietary CRISPR-based gene editing platform to treat herpes type 1 and type 2 (advancing the development of EBT-104) and hepatitis B (EBT-107 is developing).
Excision CEO Daniel Dornbusch said, " Assessing any new molecular entity first evaluates the safety of participants, especially when exploring the potential of new treatment models based on CRISPR therapy. As a first-in-human trial, EBT-101-001 was designed to assess the safety and tolerability of systemic administered CRISPR administration. The primary endpoints of the study are safety and tolerability, and the secondary endpoints are biodistribution and immunogenicity.”
"We know that many people want the first trial will provide evidence of a possible cure for AIDS, because the field has been waiting for more than 20 years. However, this clinical trial must determine the safety of EBT-101 as a gene therapy product and the safety of CRISPR use in this field, " said William Kennedy, MD, senior vice President of Excision, Clinical.
Professor of Medicine, Washington University School of Medicine, St. Louis, Phase 1 / 2 investigator, MD Rachel M. Presti added: "" Preliminary data from the EBT-101-001 trial provide important clinical evidence that gene-editing therapy modalities can be safely used to target HIV DNA pools in human cells. This study provides researchers with valuable insights into how to apply CRISPR technology to treat infectious diseases and is an important first step towards other initiatives designed to optimize this treatment modality to treat millions of patients affected by HIV and other infectious diseases.”
sum up
Born in 2012, CRISPR gene editing technology is probably the most concerned scientific breakthrough in the life sciences since the 21st century. In December 2023, the US FDA approved the first CRISPR-based gene-editing therapy, Casgevy (exa-cel), for the treatment of sickle cell disease (SCD). In February 2024, the EU also approved the Casgevy, which is the first approved CRISPR gene editing therapy in the EU, indicating that CRISPR can truly meaningfully solve the challenging problems that patients face.
At the same time, a number of CRISPR gene editing therapies are active around the world.
Globally, the layout of CRISPR companies are CRISPR Therapeutics, Editas Medicine, Intellia Therapeutics, Caribou Biosciences, Beam Therapeutics and so on. Among them, NTLA-2001 of Intellia is the fastest advanced in vivo gene editing therapy for CRISPR for the treatment of thyroxin transporter amyloidosis.
Domestic boya gen, guide gene, technology, ruifeng, bang yao biological, new bud gene have layout, including the guide gene BD111 for CRISPR antiviral gene editing drugs is the first approved into clinical gene editing drugs, development of CRISPR / Cas 9 gene modification BCL11A red enhancer of autologous CD34 + hematopoietic stem progenitor cell injection ET-01 is China's first approved by the state food and drug administration for clinical trials of gene editing therapy and hematopoietic stem cell therapy in development.
In the field of disease, the application scope of CRISPR gene editing therapy is expanding from the treatment of genetic diseases to chronic diseases, such as cardiovascular disease and HIV infection, which is expected to show a wider application prospect in the future.

Photo source: https: / / innovativegenomics.org/
References: https: / / www.biospace.com/news/




