research background
Years of experience in the pharmaceutical industry tell us that the road to drug development is tortuous and long, so that every drug molecule seems to have a "tough determination" story. However, due to high failure rates in drug development, it is estimated that only 1 in 10,000 compounds can enter the market; most of their stories are never told, only overwhelmed by heavy historical dust.
For a few lucky molecules, in the process of converting molecules into drugs, including identification and quantification of crystalline solid forms, characterization of molecular structure and properties, purification, storage and preparation development of crystallization, a lot of information on the solid form and its properties. Blockbuster drugs will also attract the attention of generic drug companies, seeking the early entry of generic drugs to the market by avoiding the original crystal type patent or making the original crystal type patent invalid, and this process may cause a new round of crystal type research on the drug. Of course, the development of commercially molecules is also accompanied by the simultaneous development of new experimental design and computational methods, and technological innovation may allow us to see a different crystal world.
In the early stages of development, most candidates will be screened to identify viable crystal forms (generally indicated temporarily), and the solid forms of drug candidates with potential for commercial development will be more fully studied. In this process, the compounds may be presented in multiple solid forms (such as polycrystalline (stable / substable, anhydrous / hydrous / hydrate / solvent compound), salt / cocrystalline and amorphous, etc.), but limited by technology and characterization equipment, not all solid forms can be discovered and deeply studied. This paper introduces the development of solid-state drug with olanzapine as a case, and mainly introduces the complexity of crystal type research and the important application of technology in crystal type characterization.
Case sharing: Olanzapine crystal type development
Olanzapine (OZPN) is an atypical antipsychotic, initially marketed under the trade name Zyprexa, for the treatment of bipolar disorder and schizophrenia. In the early stages of its development, it was shown that OZPN could crystallize itself, easy to crystallize and highly crystallizable, but also particularly easy to form solvation with various solvents. In many crystalline forms, two anhydrous polymorphic types, the crystal types I and II, were identified. Crystalline II is usually obtained by dessolvation, while Crystalline I is directly obtained from a solution that is not solvated with OZPN and contains relatively little solvent. Crystal I is the most stable crystal thermodynamically with excellent solid performance (thermal stability, solubility, moisture absorption and morphology, etc. In the commercial development stage, crystal I is applied in the market, but the discovery and development of OZPN crystal is not smooth.
In the mid-1990s, long after forms I and II were discovered (and patented), it was determined that the metastable form (form II) was a mixture of two metastable forms (hereafter referred to as forms II and III). Although, in principle, they can be identified using any technique that can distinguish crystal forms, not all techniques can reach the same conclusion, as mentioned above: common solid-state analysis techniques have different sensitivities to molecular arrangement and sometimes require combinatorial characterization of analytical tools. In the early stages of development, from the XRPD, FTIR or DSC results for characterizing the OZPN crystal form, the presence of phase III impurities was not obvious. Until the application of the solid-state 13C NMR spectrum, it found clear differences between different batches of "crystal II", which could only be attributed to the presence of different amounts of solid-phase impurities. As shown in Figure 2 (a), the ssNMR profiles of crystal type II / III batches (A and B) are strikingly similar, but at 15-20 and 115-120p.p. The relative peak intensities at m are clearly different.
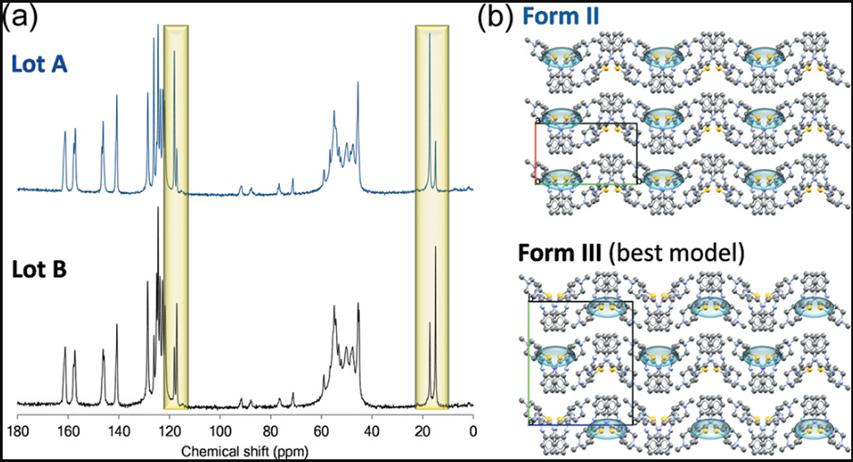
Figure 2. (a) Solid state of two OZPN II / III mixtures (batches A and B)13C NMR Spectra. Regions showing different relative peak intensities are highlighted, the clearest indication of the phase mixture.(B) crystal structure view of type II (CSD code: AQOMAU03) and CSP closest to type III (structure A162). The outward-facing thiophene S atoms (yellow) are highlighted to emphasize the stacking differences between the experimental crystal type II and the calculated crystal type III crystal structures.1
Since type III single crystals were unable to be cultured for structure determination, an attempt was made to identify the structure by two-phase Pawley-type refinement. The lattice parameters and crystal structure prediction (CSP) studies of the crystal type II crystal structure and the best available crystal type II / III mixed phase PXRD map were refined (refinement). The final crystal type III structural model combined with the crystal type II provided the best statistical fit, but does not exactly match the PXRD pattern (refinement did not consider some peaks assumed to be crystal type III). However, it appears to be close enough to characterize Form Form III as a hierarchical variant of Form II (layered variation), see Figure 2 (b). This is important to understand why dessolvation always leads to the concomitant formation of crystal forms II and III.
The commercial success of Zyprexa has provided a huge impetus for the generic drug industry, with generic scientists working to find new crystal forms of OZPN or ways to bypass known (patented) forms. Along these lines, efforts are made to produce products with metastable forms to render stable form patents invalid and to search for completely different solid forms that can be used to provide OZPN to patients. Reports on new polycrystalline forms of OZPN soon appeared in the scientific and patent literature, which, if true, would increase the total number of known pure OZPN polycrystalline forms to at least six. Unfortunately, the claim of the new crystal form is not actually correct, based on the misunderstanding of the PXRD understanding of the physical mixture. Some publications, as well as the patent claim of the new OZPN crystal type II, ignored or ignored the 2003 report (identified the mixture in the crystal type II with the crystal type II), and in the 2011 publication acknowledged the existence of the crystal type III, reported the newly discovered crystal type IV, the PXRD profile of the single crystal structure and crystal type II, quickly refuting the claim of the new crystal type. It was not until recently in 2020 that OZPN crystal type IV was confirmed.
Olanzapine is a benzodiazepine compound and is the second generation atypical antipsychotic, mainly used for the treatment of schizophrenia and related psychiatric disorders. According to the biopharmaceutical classification system, olanzapine belongs to the class of drugs, that is, it has low solubility and high permeability, and the crystal form and co-crystal of olanzapine have been widely studied. According to the University of Cambridge crystal database (Cambridge Crystallographic Data Centre, CCDC), olanzapine has reported 4 crystal forms, 56 solvent compounds and 4 kinds of co-crystal. Of course, the path to solid-state research on olanzapine continues.
sum up
Through the perspective of OZPN highlighted the importance of crystallographic chemistry in drug development. Taste OZPN research, can present a relish story of crystal development, but it is worth to believe that collective understanding of OZPN solid chemistry will continue to develop, and each for solid state research and development, newcomers to read the story, the drug molecules surprisingly complex crystal chemical images will always shine in the long river of history. What kind of inspiration can the above give us again?
1) From the perspective of product development, the goal is to develop products with stable bioavailability for clinical needs and process development feasibility, all of which are related to the solid form of the drug. In the drug discovery and development stage, it is necessary to pay attention to the screening and characterization of the solid form of drugs, establish the connection between the solid form of drugs and the key quality parameters of the product, and control the solid form of drugs to ensure the continuous production of products that meet the quality standards.
2) Carry out drug design and preparation development according to the drug nature and disease characteristics. The general goal of solid state development is solid state dominant crystal type, which is characterized by good stability to ensure the consistency of solid state form of products between different batches. However, in order to meet certain development needs, sometimes other forms of solid-state screening, such as amorphous solid dispersion, are needed to solve the solubilization problem of insoluble drugs. This also makes the forms of solid state screening more diverse to meet the needs of different solid state forms.
3) The crystal structure determination of small molecule drugs is the foundation of the drug substance industry. Traditional crystal structure determination methods rely on X-ray diffraction (X-ray diffraction, XRD) technology, such as single crystal X-ray diffraction (single crystal X-ray diffraction, SCXRD) and powder X-ray diffraction (powder X-ray diffraction, PXRD or XRPD). Similar to the OZPN crystal type II and crystal type III mixed crystal problems, the traditional characterization means cannot distinguish, MicroED can analyze the structure of different crystal components in the mixture. It is worth believing that technology can let us see more beautiful.
reference documentation
1.Rantanen J, Rades T, Strachan C.Solid-state analysis for pharmaceuticals: Pathways to feasible and meaningful analysis.J Pharm Biomed Anal.2023 Nov 30;236:115649. doi: 10.1016/j.jpba.2023.115649. Epub 2023 Aug 19. PMID: 37657177.
2.Reutzel-Edens SM, Bhardwaj RM.Crystal forms in pharmaceutical applications: olanzapine, a gift to crystal chemistry that keeps on giving.IUCrJ.2020 Oct 3;7(Pt 6):955-964. doi: 10.1107/S2052252520012683. PMID: 33209310; PMCID: PMC7642794.
3. Tan Zhongchuan, Yang Desen, Gan Guoping, etc. Crystal structure of a new olanzapine methanol hydrate and Hirshfeld surface analysis [J]. Medical Guide, 2022,41 (02): 235-239.




