For a long time, API solubility, low bioavailability is a big problem for drug developers, low solubility, lead to its preparation development form limited, to the preparation of scientific researchers brought great challenges, of course, it will greatly affect the clinical curative effect and severely limit its further development and clinical application. As a preparation research and development personnel, is to try every means to use the preparation means to "impossible" into "possible", of course, difficult to cook without rice, or with the help of new technology, new accessories, new equipment. The rise of nanotechnology has brought dawn to the development of insoluble drugs, combining nanotechnology and pharmacy, nanodrugs, namely drug-carrying nanoparticles (such as nanoparticles, 1 ~1 000 nm) or nanodrug crystals (drug nanocrystals, the nanolization of the drug itself). Drug-loaded nanoparticles refers to the drug wrapped or adsorbed into the carrier (the carrier can be liposomes, nano-gold, micelles, etc.), the preparation process is complicated, such as low drug load and poor stability; The drug nanocrystal technology is a pure drug nano-colloidal dispersion system, the drug itself, without the load of carrier material, simple preparation process and high drug load. Nanocrystals technology in the past 30 years has made great development in the field of pharmacy, preparation technology constantly updated, according to domestic and foreign reports has shown a variety of nanocrystals in clinical widely used, such as anticancer drugs, antifungal drugs, hormone drugs, anti-inflammatory drugs, and as an intermediate preparation technology can act on a variety of forms, such as oral, gastrointestinal administration, lung administration, injection and eye administration, etc.
Nanocrystals provide a feasible technical approach to improve the administration of insoluble drugs. This technology reduces the particle size of the drug to the nanoscale. When the drug exists in the nano size, the properties of the drug can also change "dramatically", which can effectively improve the solubility and dissolution rate of the insoluble drug, reduce the drug volume, reduce the toxic side effects, so as to improve the bioavailability and clinical efficacy. For example, the first sirolimus tablet, marketed in China in 2000, is 21% more bioavailable than the liquid preparation (liposomes oral), which is more convenient to take. However, the decrease of the nanocrystalline particle size is caused by the decrease of the high energy particles, the surface free energy of the particles increase one or several kinds of stabilizer (crystal growth inhibitor). Some commonly used stabilizers include: povidone, phospholipids, polysorbate, poloxamer, cellulose, or anionic surfactants. The mechanism of action of stabilizer is mainly through the electrostatic repulsion between ions.
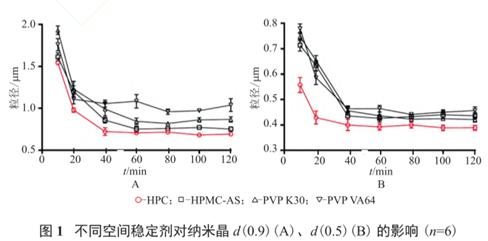
For example, in the preparation of ibuprofen nanocrystalline preparation, SDS was selected as ion stabilizer, and HPC, HPMC-AS, PVP K30, and PVP VA64 were selected as spatial stabilizers to investigate the effect of different groups of stabilizers on the product particle size. The results showed that HPC, as the space stabilizer, stabilized the drug particle size the best, so HPC-SDS was selected as the combined stabilizer.
1. Drug preparation method of nanocrystal crystals
The nanocrystals were prepared mainly by Bottom up and Top Down. Top Down Method, also known as dispersion method, is to reduce the particle size dispersion of drugs by mechanical forces such as grinding or homogenization. It mainly includes the media grinding method and the high-pressure homogenization method. This method has good reproducibility, easy to process and further industrialization, and is a common means of nanocrystalline preparation.
1) Top Down-media grinding method
Medium grinding method can be divided into wet grinding and dry grinding two kinds. Wet grinding is the mixing of polymeric materials such as drugs and stabilizers in water, And then it was added to the medium grinder, Media grinder should be added in advance grinding media (grinding media generally with steel beads, glass beads, ceramic beads, zirconia, agate, etc.), After the instrument is started, there is a strong collision and shear force between the drug particles, the grinding medium and the device wall, So that the solid particle size gradually decreases to the nanometer scale, When the particle size is smaller than the filter gap of the grinding chamber separator, Exude the grinding cavity by centrifugal force into the material cylinder, Monitor the change of particle size in real time at certain intervals, If not met, Until the particle size meets the required requirements. Dry grinding is a direct dry grinding of drugs and stabilizers to obtain nanocrystals with the desired grain size. The disadvantage of dry grinding is the dust and adhesive wall, which is not conducive to industrialization, and the nanocrystals made by wet grinding are stable and easy to treat.
In general, the key material attributes CMA (API concentration, API initial particle size, stabilizer type and add amount), key process attributes CPP (grinding medium material, beads particle size, material circulation flow rate, cycle time, feed quantity, temperature) to the nanocrystalline key quality attributes (size distribution and change, physical stability, crystal, ζ potential, chemical stability), etc, so in the preparation process, to real-time monitoring these parameters may change, to meet the requirements and stable nanocrystalline drugs. Generally in the preparation process, select the appropriate API concentration, minimize the initial particle size of API, choose the appropriate stabilizer and quantity, micro bead size selection (the smaller the bead size, the same weight number and contact point, the higher the collision frequency, the better the grinding effect), speed set reasonable (the higher the speed, the faster the particle movement, the higher the grinding efficiency), control these conditions can make more ideal nanocrystalline drugs.
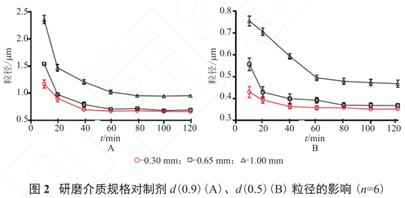
For example, during the preparation process of ibuprofen nanocrystalline crystal, the influence of three different specifications of zirconia beads (d=1.00,0.65,0.30mm) on the grinding effect was investigated. With the extension of the grinding time, the particle size trend of the three specifications is flat, almost reaching the minimum particle size of the grinding system at 60min. When choosing 0.3mm grinding beads, it can reach a smaller particle size in a short time, so we choose 0.3mm grinding beads as the grinding medium.
2) The TopDown-high-pressure homogenization method
This method is considered to be the second most commonly used technique for nanocrystalline drug preparation. The action principle of this method is: API + stabilizer suspension is imported into the homogenization valve by high pressure pump, the instantaneous pressure loss material at a very high flow rate, and collide on the collision ring of one of the valve components, producing high speed shear, impact and hole three effects, to achieve the role of refinement and homogenization. The nanocrystalline drugs prepared by this method have small particle size, narrow particle size distribution and good reproducibility. Key material attributes CMA (initial particle size, API concentration, stabilizer type and dosage before API homogenization) and key process attributes CPP (homogenization pressure, cycle times) will directly affect the quality attributes of nanocrystalline drugs, and may affect the particle size, ζ point, crystal shape, dissolution results of the final nanocrystalline products.
3) The Bottom up method
Also known as reverse solvent precipitation method, it is to first dissolve the drug in a good solvent, and then add it to another bad solvent that can be miscible. Through the change of solvent, the drug concentration oversaturate and precipitate the crystal of the drug by controlling the formation and growth rate of the crystal core. The preparation process of this method is simple, the experimental cost is low, the process is easy, but the disadvantages are also obvious, the organic solvent is used in the test process, which is easy to cause organic solvent residue and toxicity problems. In this method, the induced formation and growth of the crystalline core is the critical rate-limiting step in the crystallization process. Figure (a) is the crystallization induction stage; (b) - (c) core formation period; (c) - (d) core growth period; and the type and concentration of stabilizer, crystallization temperature, solvent selection and solvent are the key quality properties affecting core growth. Generally, the more core formed, the smaller the crystal size.

Figure: Core and crystal size observed under a polarized light microscope
2. Research on the preparation process of nanocrystalline drug preparation
By Bottomup or Top Down method of nanocrystalline drug is just a preparation intermediate, after the preparation often exist in liquid form, and we need to do further processing, its further converted into oral solid preparation (tablet or capsule), the purpose is to improve the stability of the drug, improve patient compliance and treatment effect. So how to effectively transform it into commonly used tablets or capsule dosage forms is a difficult problem in research and development.
To develop an ideal tablet or capsule dosage form, ensure that the nanocrystalline intermediate maintains good physical and chemical stability: the nanocrystalline intermediate must keep the same size and the preparation size. The good physical and chemical stability of nanocrystalline intermediates can be guaranteed by controlling the prescription screening of nanocrystalline preparation, and the control of the compatibility study of excipients and API in the early stage of innovative drugs.
How to successfully convert nanocrystalline intermediates into stable solid preparation? Spray drying, fluidizing bed top spray granulation and coating technology are common means. Of course, the process difficulty of transforming nanocrystalline drugs into solid preparations is higher than that of ordinary preparations. First of all, nanocrystallized drugs are mostly suspension intermediates with high drug load, and a large number of excipients are added to ensure their physical stability, so the viscosity and solid content are high. The high proportion of weight increase makes the process time long and the parameters change greatly; Secondly, if select the wet weight of fluidized bed, the nanocrystalline drug may accumulate and affect the particle size; if select the dry weight, the improper pressure may change the structure of the nanoparticles, and the pressure adjustment will also affect the crystal structure...... In order to avoid the impact of tablet pressure on the crystal, some products will choose granule capsule to reduce the impact of pressure on nanoparticles, and some products will wrap the nanocrystalline intermediate on the surface of the blank tablet core in the form of coating to completely avoid the generation of pressure. So in the early stage of research and development or as detailed as possible to explore preparation prescription process, including key material properties, key process parameters, key quality attributes to do detailed investigation and exploration, undoubtedly increase the workload of research and development personnel, but there is no retreat, preparation development personnel can only carry burden to open the way, will be "breakthrough", usher in victory.
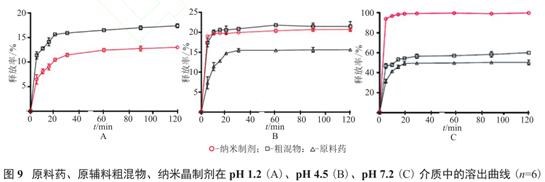
For example, in the preparation of ibuprofen crystals, the dissolution curve of 0.2gAPI, raw material and nanocrystalline was put into 900ml of pH1.2,4.5 and 7.2 media, respectively. The results showed that in PH 7.2 medium, nanocrystalline can be completely dissolved within 10min, and raw materials and API only dissolved by 60.4% and 30.7% respectively within 2 hours, which further showed that the optimization of particle size can enhance the solubility of the drug and improve the dissolution rate.
to sum up, Although nanocrystalline technology are effective in improving the solubility of drugs, To improve the patient bioavailability, Has a very broad development prospects, But it must be realized that the technology is not yet fully mature, There is still something to be perfected, For example, nanocrystalline are in a high-energy state in gastrointestinal fluid, Belongs to a thermodynamically unstable system, If there is no crystallization inhibitor in the prescription or an improper selection of type, concentration, Although the drug can also crystallize very quickly, But it will quickly reduce its own energy through further aggregation, agglomeration, etc., To reach a stable low-energy state, The phenomenon is like a spring process; And if the prescription contains a reasonable selection of polymeric crystallization inhibitors, The crystallization precipitation of the drug will be slow, Like a parachute, The energy has also dropped very slowly, Thus, maintaining the supersaturation concentration for a long time allows the nanocrystalline drug to maintain good homeostasis. Based on this, we need to further strengthen the research on the key factors affecting the preparation and stability of nanocrystalline drugs, try to optimize the preparation process of nanocrystalline drugs, further reduce the particle size and improve the stability of nanocrystalline drugs. At the same time, we need to study and explore the in vitro and in vivo relevance and safety evaluation of nanocrystalline drugs. Of course, our ultimate goal is to return to the original intention: to improve drug solubility through the means of preparation, to bring better efficacy to patients.
[reference documentation]
1. Study on the prescription process of insoluble drugs
2. Application of high pressure homogenization in the preparation of nanopreparations
3. Nanodrug crystal technology: "timely rain" to improve the solubility of insoluble drugs
4. The success of nanotechnology in generic drug development is the key
5. Review and prospect of 30 years of drug nanocrystal preparation technology
6. Research progress in nanocrystal drugs
7. Progress and thinking on the application of nanocrystal technology in insoluble drugs
8. Development of ibuprofen nanocrystalline drug preparation




