T lymphocytes play a crucial role in the adaptive immune responses. They recognize and activate the antigen through surface antigen receptors, initiating a series of signaling and metabolic changes to support proliferation, differentiation, and effector functions. Dysregulation of T cell metabolism is closely related to a variety of diseases (such as cancer and autoimmune diseases).
Overview of metabolic pathways
Glycolysis and pentose phosphate pathway (PPP): Glycolysis breaks down glucose into pyruvate, producing a small amount of ATP but generating important intermediates for cell growth and function. PPP to generate the NADPH and the nucleotide precursors.
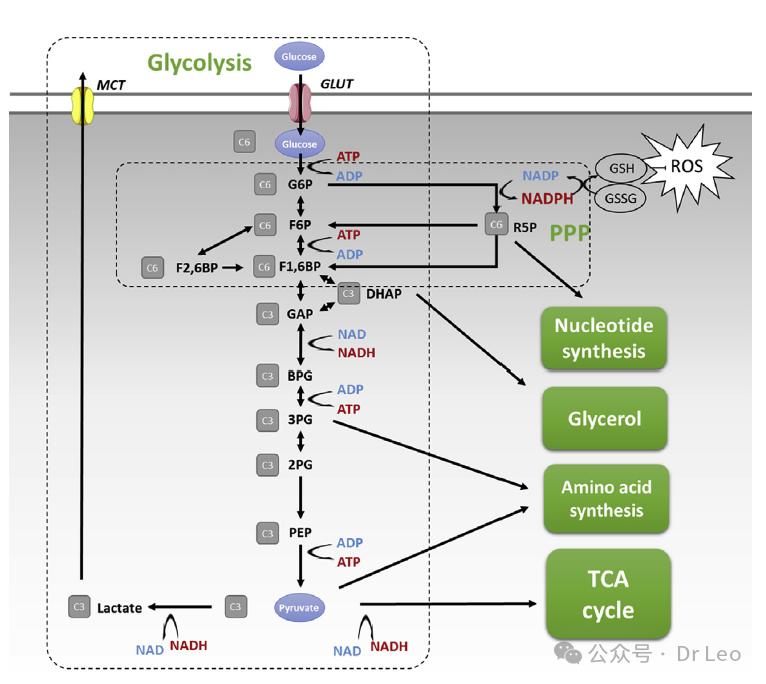
The tricarboxylic acid cycle (TCA) and oxidative phosphorylation (OxPhos): TCA is performed in the mitochondrial matrix, linking glycolysis, fatty acid oxidation and amino acid decomposition to generate the high-energy molecule ATP.
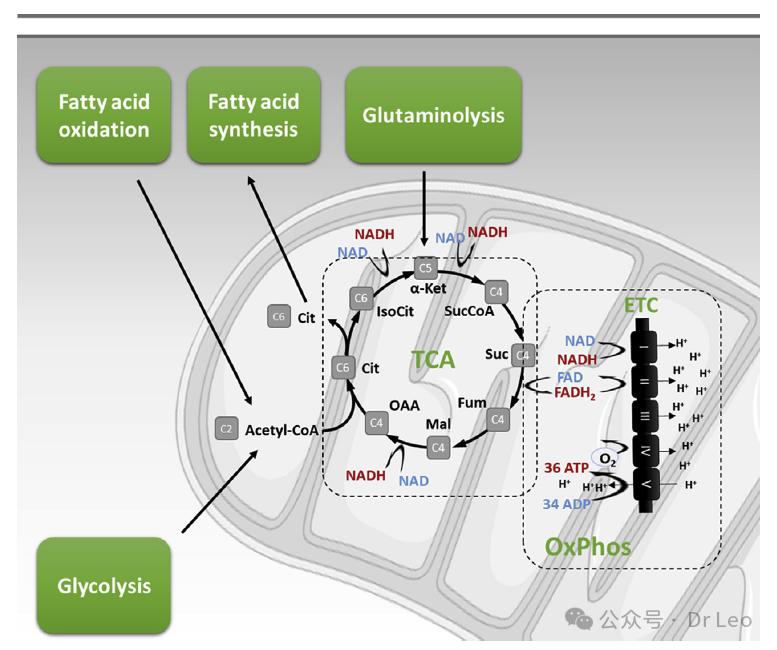
Fatty acid synthesis and oxidation: Fatty acids are synthesized within the cell and provide energy through oxidation. Different types of fatty acids are involved in cell membrane and signaling molecule synthesis.
Amino acid and glutamine metabolism: Amino acids are the basis of protein synthesis, and glutamine provides energy and intermediates through glutaminolysis.
Specific metabolic characteristics of T cells
CD8 + T cells: predominantly differentiated into cytotoxic T lymphocytes (CTLs), dependent on glycolysis and oxidative phosphorylation.
CD4 + T cells: they can differentiate into multiple effector T cell subsets (such as Th 1, Th 2, Th 17, Tfh) and regulatory T cells (Treg), each with unique metabolic characteristics.
Metabolic pathway for maintaining T cell homeostasis
Resting T cells: using low levels of glycolysis and fatty acid oxidation to maintain basic cellular functions. They mainly generate ATP through oxidative phosphorylation to maintain cellular homeostasis.
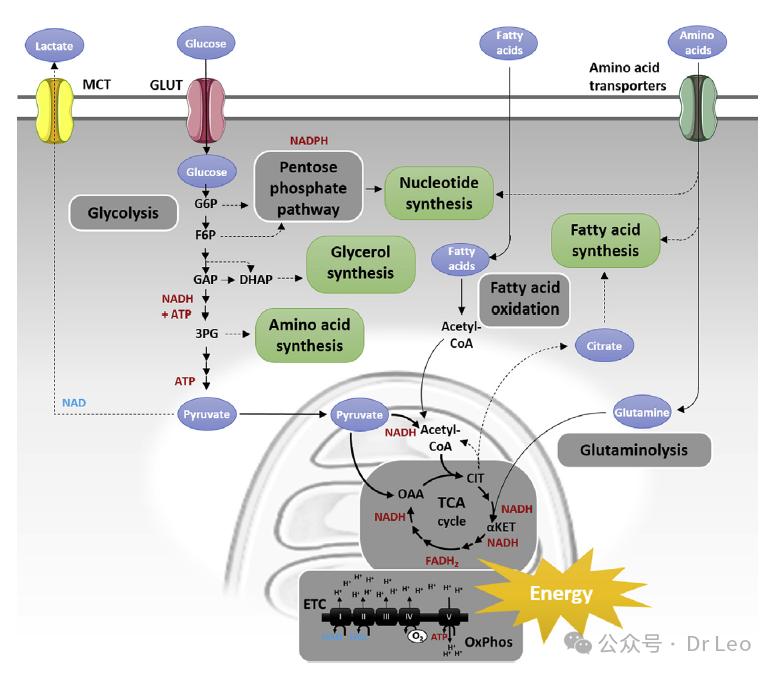
Overview of the metabolic pathways in resting naive T cells. Long-lived and resting T lymphocytes have minimal requirements for bioenergy and biosynthesis besides maintaining basic cellular functions. Therefore, initial T cells use low levels of glucose-derived pyruvate, glutamine-derived α -ketoglutarate (α -Ket) and fatty acids to supply the TCA cycle (TCA) to generate reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH 2) and energy adenosine triphosphate (ATP) via electron transport chain (ETC) and oxidative phosphorylation (OxPhos) of adenosine.
Metabolic reprogramming of effector T cells
Effector T cells require large amounts of energy and synthetic intermediates after activation, and metabolism shifts from primary ATP production to producing intermediates of new cellular components. Under the regulation of TCR and CD28, effector T cells significantly increase the activity of glycolysis and PPP to support rapid proliferation and effector functions. The mTOR is a key regulator of the metabolic reprogramming of effector T cells, promoting cell growth and differentiation by regulating glycolysis and PPP.
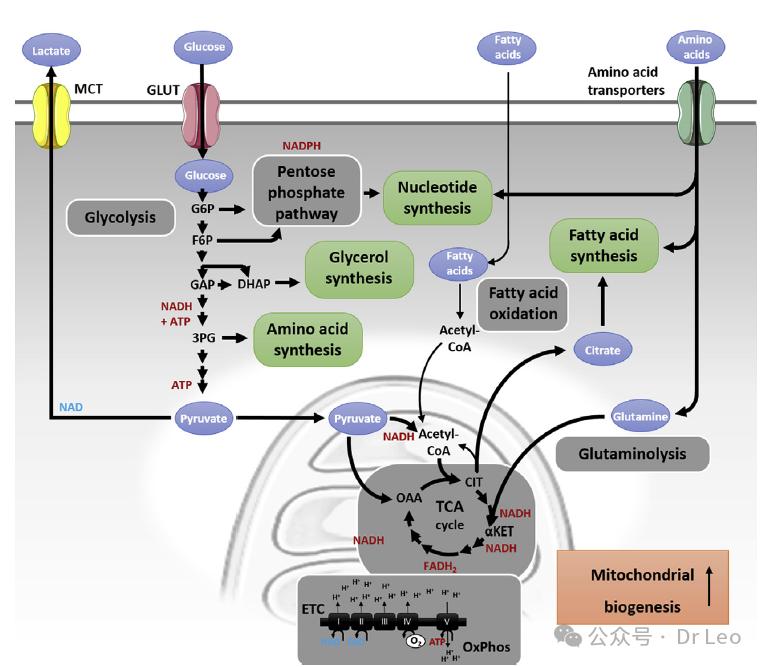
效应 T 细胞在 T 细胞抗原受体(TCR)结合后代谢的重构。作为分化、增殖和获得完全效应功能的关键事件,T 淋巴细胞经历了从氧化代谢到有氧糖酵解的显著代谢转变,这也被称为沃伯格效应。有氧糖酵解的进行增强了核苷酸、氨基酸、甘油和脂肪酸合成所需中间体(构建模块)的合成,同时适量的腺苷三磷酸(ATP)仍通过三羧酸循环(TCA)、电子传递链(ETC)和氧化磷酸化(OxPhos)生成。糖酵解代谢产物为互联途径提供燃料,例如磷酸戊糖途径、核苷酸合成、甘油和氨基酸合成途径,以及通过乙酰辅酶 A(acetyl-CoA)使用的丙酮酸。乙酰辅酶 A 被输送到 TCA 循环中,用于脂肪酸合成和在 OxPhos 中的使用。伴随着葡萄糖代谢的变化,TCR 的结合还增强了线粒体生物合成和 OxPhos,推动线粒体膜超极化、氨基酸摄取和谷氨酰胺分解。

The metabolism of resting initial T cells (Tn cells), activated T cells, and the metabolic changes of T cells during activation (initial) and differentiation involve their different subtypes. For clarity, the differentiation of naive T cells (Tn cells) into regulatory T cells (T reg) and memory T cells (Tmem) is not shown here. Unlike Tmem or Tn cells, Treg cells continuously proliferate at intermediate levels.
Immunometabolism of regulatory T cells (Treg)
Treg cells depend on fatty acid oxidation and low levels of glycolysis. As a key transcription factor, FoxP3 regulates the metabolic status of Treg cells, suppresses the PI3K-Akt-mTORC1 axis, and limits GLUT1 expression and glucose uptake. Treg, cells generate ATP via FAO, promoting their differentiation and function. Low mTOR activity favors the maintenance of the oxidative metabolic state in Treg cells.
Metabolic remodeling and persistence of memory T cells
Memory T cells maintain high mitochondrial quality and spare respiratory capacity (SRC) and respond rapidly when encountering the antigen again. Memory T cells respond rapidly to infection through the fatty acid synthesis and oxidation cycle (ineffective cycle). It depends on newly synthesized fatty acids rather than exogenous fatty acids.
Effects of the tumor microenvironment on T cell metabolism and function
Nutritional competition, hypoxia, and acidosis in the tumor microenvironment significantly affect T cell metabolism and function. Understanding the effects of the tumor microenvironment on T cells could facilitate the development of therapies that enhance the antitumor immune response.
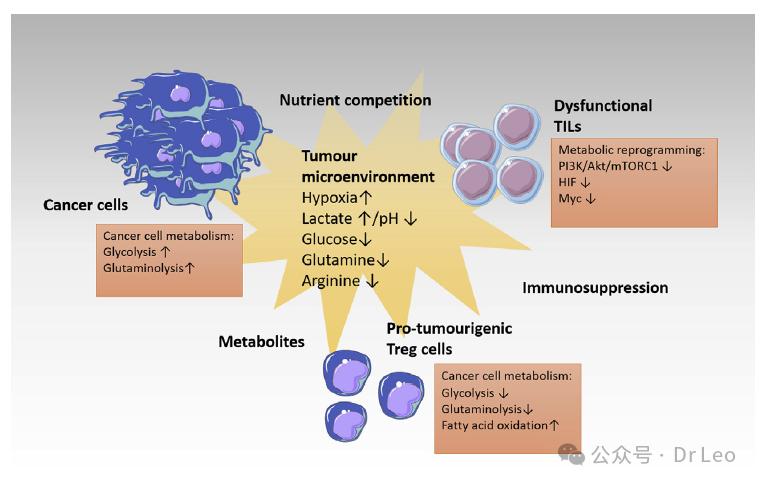
The tumor microenvironment that shapes T cell metabolism and function. Cancer cells and paraxtostromal cells with high glycolysis and high glutaminolysis establish the surrounding microenvironment and compete with tumor-infiltrating lymphocytes (TILs) for nutrients, while promoting the differentiation and recruitment of regulatory T cells (Treg cells) through their metabolites. This ultimately leads to a dysfunctional antitumour lymphocyte response.
The metabolism of T cells in the autoimmunity
Such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), the metabolic dysregulation of T cells is closely related to disease progression. By reprogramming T cell metabolism, it may become a new approach to treat autoimmune diseases.
inspiration
T cells need to undergo metabolic reprogramming upon activation to support rapid proliferation and effector functions. Understanding this process could facilitate the development of strategies to enhance the immune response. T cell metabolism not only supports its energy requirements, but is also directly involved in immune regulation. It is important to study the mechanism of regulating immune response. Dysregulation of T cell metabolism is associated with the pathophysiology of multiple diseases. By regulating T cell metabolism, new therapeutic approaches can be explored to control and treat these diseases. The inhibitory effect of the tumor microenvironment on T cell metabolism and function suggests the need to develop novel approaches to enhance the antitumor immune response. Different types of T cells differ significantly in their metabolic requirements, and metabolic regulation targeting specific T cell subsets may provide new avenues for personalized therapy.




