preface
Regulatory T cells (Treg) are a small fraction of immune cells that are designed to inhibit excessive immune activation and maintain immune homeostasis. Over the past two decades, the development of chimeric antigen receptor (CAR) and advances in genome editing have facilitated the optimization of T cell therapy, and these techniques are now used to enhance the specificity and functionality of Treg cells, and Treg cell-based autoimmunity and transplant therapies are being rapidly developed.
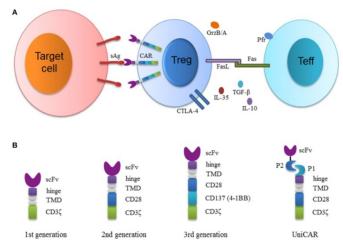
Classification and function of Treg
Treg, a subset of T cells representing 5 – 10% of total CD4 + T cells, has a function of maintaining homeostasis and preventing autoimmunity and is characterized by co-expression of CD4, CD25, FOXP 3 and low levels of CD127. High levels of FOXP 3 and demethylation of specific demethylated regions (TSDR) are prominent features of Treg, and TSDR is a conserved region in the FOXP 3 gene.
Tregs can be divided into two categories: natural regulatory T cells (nTregs) and induced regulatory T cells (iTregs). Foxp 3 is ubiquitously expressed by Tregs of both types. nTregs Developed naturally in the thymus, its inhibitory effect is achieved by intercellular contact, and their main function is to maintain normal immune tolerance and control inflammatory responses.
iTregs Are derived from peripheral primitive T cells induced by tumor microenvironment signals, including tumor antigens, cytokines (e. g., IL-10, TGF- β), and other soluble molecules.iTregs Inhibit the anti-tumor immune effects of effector T cells (Teff), NK cells, and DC through various mechanisms that promote tumor progression.
Tregs mainly have the following five functional mechanisms: ① Tregs secrete inhibitory cytokines, including IL-10, TGF- β, and IL-35. ②Tregs Killing of effector cells by granzyme and perforin.③ Tregs Affects the function of effector cells: Treg competes with effector T cells to consume IL-2, thus inhibiting the growth of effector T cells; Tregs promotes the production of adenosine in the TME by producing the extracellular enzymes CD39 and CD73, inducing the inhibitory and anti-proliferative effects of effector cell guides; Tregs transfer a large amount of cAMP to effector T cells through gap junctions and interfere with their metabolism.④ Tregs induce DC tolerance through stimulatory and inhibitory receptors (CTLA-4 or LAG 3), which further inhibit the ability of T cells through IDO. Factors produced by ⑤ MDSCs and Tregs form a positive feedback loop that promotes proliferation and enhances the inhibitory environment.

Furthermore, one study used a new strategy to define Tregs, Th 1-like Tregs (T234bet + IFN γ + Foxp 3 +), Th 2-like Tregs (Gata 3 + IRF 4 + IL 4 + Foxp 3 +) and Th 17-like Tregs (IL-17 + ROR γ t + Foxp 3 +), which provided new ideas for targeted Tregs therapy.
Adoptive Treg: from polyclonal to antigen specificity
Initial clinical trials of adoptive Treg used polyclonal or ex vitro expanded Tregs. The first report on GVHD treatment with ex vivo expanded Treg suggests that symptoms resolve in chronic GVHD patients and has the potential to reduce the use of immunosuppressants. A few years later, 11 GVHD patients who received stem cell transplantation for hematological malignancies obtained cord blood-derived Treg and were amplified and administered. Patients receiving Treg had a five-fold reduction in the incidence of grade II – IV acute GVHD, and the Treg-treated group was free of chronic GVHD after one year, compared to 14% in the control group.
Although polyclonal Tregs achieve some encouraging effect, the number of cells required for infusion is considerable and, in addition, there is a risk of nonspecific immunosuppression, and in fact, viral reactivation after polyclonal Tregs infusion has been reported.
These drawbacks can be overcome by the use of antigen-specific Treg, which requires only fewer cells to perform more local and targeted inhibition compared to polyclonal Treg. Moreover, many studies have demonstrated that Treg specific to the desired antigen is functionally superior to polyclonal or unmodified T reg in animal models.
Traditional methods to produce antigen-specific Treg mainly rely on amplification with APCs and specific antigen, or engineering of Treg with T cell receptors (TCRs). However, Treg amplification with APCs is inefficient, while Tregs (TCR-Tregs) constructed with TCR are still limited by the MHC, limiting modular applications for different patients.
Design of the CAR-Treg
Another way to confer specificity to Tregs is transduction of these cells with CAR. CAR has some unique advantages over TCR-Treg: these T cells expressing CARs bypass HLA restriction upon activation, increased specificity through activation of coreceptor signaling, and targeting flexibility of CARs (any soluble or surface multivalent antigens can target).
The most direct applications of CAR-Treg cells are GvHD and organ transplant rejection. Unlike most autoimmune diseases, transplantation has very well-defined targets, namely, HLA molecules. In 2016, the first reported HLA-A2 CAR Treg cells demonstrated that HLA-A2-CAR-Treg cells inhibited Teff cell proliferation and prevented HLA-A2 + PBMC mediated GvHD in an immunodeficient NSG mouse model.
New applications of CAR-Treg cells are still emerging, and B cell-targeted antibody receptor (BAR) Treg cells are a focus of recent research. Human factor VIII (FVIII) injections are used to treat patients with hemophilia A, and the development of anti-FVIII neutralizing antibodies over time leads to increased morbidity and mortality. The BAR containing the FVIII immunodominant domain (A2 or C2) as the extracellular domain was designed to target FVIII-specific B cells, and the intracellular signaling domain was still CD28-CD3 ζ. Strikingly, in vitro, human BAR-Treg cells exhibiting the A2 domain or C2 domain inhibited the production of anti-FVIII antibodies in mice immunized with recombinant FVIII.
Another important aspect of the CAR-Treg design is the choice of the co-stimulation field. The costimulatory domain used is the same as that used in conventional CAR T cells. Although conventional T cells and Treg usually express the same costimulatory receptors, the intracellular signals differ, leading to different effector functions.
To far, the co-stimulatory domains of CAR-Treg are 4-1BB or CD28. Recently, second-generation CAR-Treg with 10 different costimulatory domains (e. g., CD28, CD28 Y173F, ICOS, CTLA-4, CTLA-4 Y165G, PD-1,4-1BB, GITR, OX 40, TNFR 2) were investigated, demonstrating that superior CD28 costimulatory signaling occurs when testing functional and gene expression profiles. Use of either 4-1BB or TNFR 2 results in decreased Treg stability and function.
Progress in CAR-Treg in autoimmune diseases
GvHD
Treg therapy has become an important area of GvHD research, with most studies focusing on in vitro expanded polyclonal Treg. The first clinical trial of Tregs for GvHD showed little effect on acute GvHD symptoms but significantly relieved the symptoms of chronic GvHD and reduced the use of immunosuppressants.
Subsequent studies began using CAR-Tregs, an alloantigen-specific human Tregs designed to express HLA-A2-specific CAR. These A2-CAR Treg maintain high expression of FoxP 3, CD25, Helios, and CTLA-4 in in vitro and in vivo assays. Furthermore, they prevented xenograft GvHD in an immunodeficient mouse model.
Following the success of HLA-A2 targeting of CAR-Tregs, recent studies have focused on generating CAR-Tregs with different targeting domains, such as CD83, which prevents GvHD in mouse models. And CD19, which inhibits the production of B cell antibodies and causes the pathology of GvHD.
These promising results contributed to the first CAR-Treg clinical trial (NCT04817774) authorization in kidney transplant patients in the United Kingdom and the United States. This trial may support superior CAR-Tregs over polyclonal expansion in clinical trials, further expanding the possibility of using CAR-Tregs in other disease conditions. Other biopharmaceutical companies have followed closely, such as Quell Therapeutics, focusing on CAR-Tregs in liver transplant recipients.
diabetes mellitus
Type 1 diabetes mellitus (T1D) is an autoimmune disease characterized by insulin deficiency due to the destruction of pancreatic β -cells. Studies have shown that the immunosuppressive function of Treg is reduced in T1D patients. Infusion-amplified antigen-specific Treg showed promising results in blocking and reversing diabetes in animal models. However, isolating enough antigen-specific Treg due to its rarity in the circulation is challenging.
Therefore, the focus was on converting polyclonal CD4 + T cells transduced with the FoxP 3 gene into Treg. Furthermore, antigen can be specifically assigned to polyclonal T cells by engineered TCR or CAR techniques. Brusko The group found that Treg transduced by glutamate decarboxylase (GAD) -specific TCR inhibited the ability of antigen-specific T cell proliferation in vitro. In another study, Hull et al. transferred the islet-specific TCR to regulatory T cells and demonstrated their ability to inhibit the proliferation of CD4 and CD8 T cells in vitro. Currently, companies such as GentiBio and Abata are developing TCR engineering Treg for T1D.
arthritis deformans
Rheumatoid arthritis (RA) is the most common form of inflammatory arthritis and is characterized by synovial inflammation, hyperplasia, autoantibody production, and cartilage and bone destruction. Multiple immune cell subsets are involved in the development of RA, in which, the interaction between T cells and macrophages plays a crucial role.
Many studies have investigated the benefits of increasing Treg numbers or improving Treg function for RA. Wright et al. used ovalbumin (OVA) -specific Treg to inhibit OVA-induced arthritis by generating TCR-transduced Treg or TCR-FoxP 3-transduced CD4 + T cells. Both engineered Treg exhibited OVA-dependent inhibition of proliferation of different antigen-specific T cells by bystander inhibition. Currently, Sonoma Biotherapeutics is developing CAR Treg therapies for RA.
MS
Multiple sclerosis (MS) is an autoimmune demyelination and neurodegenerative disease caused by autoreactive T cells that recognize myelin epitopes. Preclinical studies using experimental autoimmune encephalomyelitis (EAE) models demonstrate the effectiveness of Tregs in suppressing antigen-specific autoreactive immune responses.
Fransson et al. targeted myelin oligodendrocyte glycoprotein (MOG) with CAR to create antigen-specific MOG Treg. MOG-CAR Tregs inhibited the proliferation of effector T cells in vitro. In vivo, MOG-CAR Tregs reduced disease symptoms in EAE mice and reduced proinflammatory cytokine mRNA levels in brain tissue. Later, Kim et al constructed Treg using a myelin basic protein-specific TCR derived from MS patients, and in vivo TCR-Tregs significantly reduced the disease score in the EAE-MS mouse model. The results of immunoregulatory engineered Treg in preclinical studies have facilitated the development of CAR-Treg by some biopharmaceutical companies, such as Abata Therapeutics and TeraImmune.
inflammatory bowel disease
Inflammatory bowel disease (IBD) is a disease characterized by chronic inflammation of the gastrointestinal tract, and ulcerative colitis (UC) and Crohn's disease (CD) are the most common forms of IBD. Studies have shown that an imbalance between the gut microbiota and the immune response plays an important role in IBD. Usually, intestinal inflammation is not associated with a reduction in Treg numbers. However, mice with insufficient Treg activity are more likely to develop severe colitis. Therefore, several studies have attempted to utilize the Treg inhibitory activity to maintain UC tolerance.
In one study, CAR-Tregs from transgenic mice against the known colitis antigen (TNP) inhibited the proliferation of effector T cells in vitro. In vivo, increased survival of transgenic CAR-Treg mice was observed compared with wild-type animals. Furthermore, transfer of TNP-CAR Tregs to a mouse model of colitis reduces symptoms and improves survival. A CAR-Treg cell-based therapy may be a promising and effective modality to alleviate both UC and CD.
asthma
Asthma is a chronic respiratory disease and Treg is impaired and reduced in asthma. Thus, the new approach focuses on preventing airway inflammation by transferring regulatory T cells in preclinical models of asthma. Furthermore, one study utilized regulatory T cell epitopes (Tregitopes) to induce highly inhibitory allergen-specific Treg.
Treatment with Tregitopes suppressed allergen-induced airway hyperreactivity and lung inflammation. To direct Treg to asthma-associated antigens, Skuljec et al applied CAR to isolate second-generation Treg targeting CEA from transgenic mice, CEA, a glycoprotein present on the gland epithelial surface of the lung and gastrointestinal tract. Their results showed the activation and homing of CEA-CAR Tregs in the inflamed lungs of the asthmatic mice. Furthermore, CEA-CAR Tregs improved inflammation to a greater extent compared to unmodified Tregs.
Next-generation engineering of the Treg
Synthetic Biology
Developing Treg cells as drugs for treating autoimmune diseases is not limited to the application of TCRs and CARs, and synthetic immunology has yielded a number of artificial receptors and systems that are being tested in Treg cells.
These systems include T cell antigen-coupling agents that recruit endogenous TCR complexes to non-MHC targets by ligated single-chain antibodies, CARs that can be bound and activated by soluble ligands, and separable, universal and programmable CAR (SUPRA).
cell factor
Undoubtedly, cytokines play a key role in the immune response. Treg cells constitutively express the high-affinity chain CD25 of the IL-2 receptor, efficiently depriving Teff cells of IL-2. Furthermore, modification of engineered CAR-Treg cells has converted pro-inflammatory cytokine signaling to IL-2 or IL-10 signaling to increase inhibition of inflammation.
gene editing
Several preclinical studies using CRISPR Cas 9 to edit human T cell genes have been published, including knockout of CCR 5 in CD4 + T cells to produce anti-HIV infected T cells; knockout of CD7 in CD7-CART cells because T cells themselves express CD7 to prevent phase killing; and knockout of PD1 in CD19-CART cells to improve tumor clearance in humanized mouse models.
Improve the delivery system
Currently, the fabrication of CART cells uses both retroviral and lentiviral transduction to deliver and integrate genetic material into T cells. However, these methods are time-consuming, expensive and plagued with safety problems.
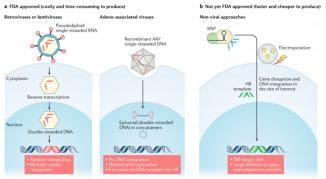
Several non-viral delivery methods are being developed, such as CRISPR-RNPs coelectroduction, which can knock more than 1 kb of DNA into specific genomic sites in human T cells, which are simple, safe, and not toxic for double-stranded DNA templates. It has corrected pathogenic CD25 mutations in the cells of patients with monogenic autoimmune diseases, but this approach is also limited by the size of the insert.
Treg cells
One obstacle to Treg cell therapy is ensuring cell survival after infusion, Tre-gene knockout of the JNK 1 gene by gene editing, and JNK 1-deficient Treg cells secrete higher levels of IL-10 and TGF β to protect transplanted islets from rejection by 100 days than in control.
In addition, Treg cells may also become unstable in an inflammatory environment and transform into pathogenic TH 17 cells. By knockdown of PRKCQ gene, the tendency of T reg cells to differentiate into TH17 cells could be reduced, while maintaining the repressive function of T reg cells.
Finally, the acquisition of the latest generation of low-immunogenic human pluripotent stem cells by gene editing, coupled with ongoing efforts to differentiate stem cells into Treg cells, may revolutionize the means of engineered Treg cell therapy.
brief summary
Currently, the design of Treg cells to treat autoimmune diseases is on the rise, and the engineered CAR-Treg shows unique advantages in this regard and has great potential. However, many problems remain in the field of Treg cell biology and Treg cell therapy.
Over the next decade, as these open questions are resolved, looking forward to see continuous improvements in Treg cell manufacturing, along with synthetic biology and gene editing, will enable Treg cell therapies to play an increasingly important role.
reference documentation:
1. The Future ofRegulatory T Cell Therapy: Promises and Challenges of Implementing CARTechnology.Front Immunol.2020; 11: 1608.
2. Chimeric AntigenReceptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance.Front Immunol.2018 Oct 12;9:2359.
3. Next-generation regulatory T cell therapy.Nat Rev Drug Discov.2019 October ; 18(10): 749–769.
4. Chimeric Antigen Receptor (CAR) Regulatory T-Cells in Solid Organ Transplantation.Front Immunol.2022; 13: 874157.
5. CAR-T Regulatory (CAR-Treg) Cells: Engineering and Applications.Biomedicines.2022 Feb; 10(2): 287.




